A*STAR NEWS
COVID-19 R&D
A*STAR & partners roll out resolute 2.0 test and automated lab system RAVE

The DSO (left) and DxD, A*STAR (right) team of scientists
Other DxD, A*STAR team members that worked on the RESOLUTE Kit
DSO National Laboratories and A*STAR's Diagnostics Development (DxD) Hub have jointly developed RESOLUTE 2.0, a breakthrough Direct-Polymerase Chain Reaction (PCR) diagnostic test kit for COVID-19. This initiative was part of a strategic partnership between DSO and A*STAR that was inked earlier this year to tighten the defence-civilian research nexus.
RESOLUTE 2.0 does not require the need for extraction of viral RNA from patient test samples. This minimises potential human errors and halves the test delivery time, compared to a conventional PCR test which takes 2.5 hours or longer.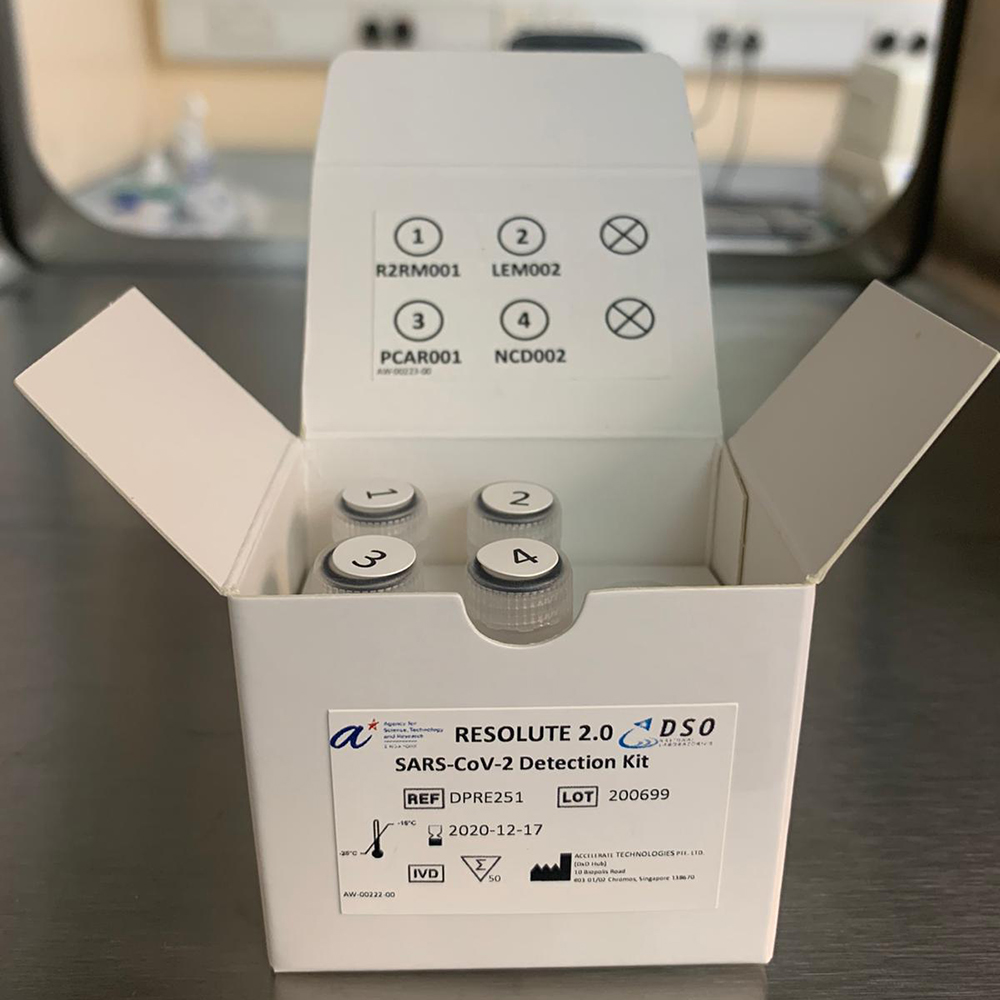
Inside a RESOLUTE test kit
To further expedite the testing process, A*STAR also developed a robotics lab system called Rapid Automated Volume Enhancer (RAVE) that complements RESOLUTE 2.0. The system automates some of the manual steps usually required in sample processing, such as uncapping of tubes and pipetting. Researchers from A*STAR’s Advanced Remanufacturing and Technology Centre (ARTC), the Singapore Institute of Manufacturing Technology (SIMTech), and the DxD Hub co-developed RAVE.

Rapid Automated Volume Enhancer (RAVE)
The integrated RESOLUTE 2.0 and RAVE system combines strong engineering capabilities with deep biomedical science, and will help to save time, manpower and costs while maintaining high accuracy and speed, and enhancing safety for laboratory staff. This integrated system can process an industry-topping throughput of close to 4,000 samples a day, 4 times the usual RT-PCR throughput.
The joint RESOLUTE 2.0 and RAVE system will be distributed by Advanced MedTech Holdings, which was also granted Provisional Authorisation from Singapore’s Health Sciences Authority (HSA) to manufacture and supply the RESOLUTE 2.0 tests. To date, the joint solution is being deployed to three hospitals in Singapore.
For licensing opportunities of the technology behind RESOLUTE 2.0, contact DxD Hub here. Parties interested in obtaining the integrated RESOLUTE 2.0 and RAVE system can approach Advanced Medtech at https://www.advancedcovidtest.com/.
Was the article helpful?
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
