A*STAR NEWS
Singapore scientists reveal a metabolic pathway as a new target in liver cancer treatment
Study showed that two metabolic enzymes in the proline metabolic pathway are potential targets to inhibit cancer cell growth, and offers new hope for effective liver cancer treatments
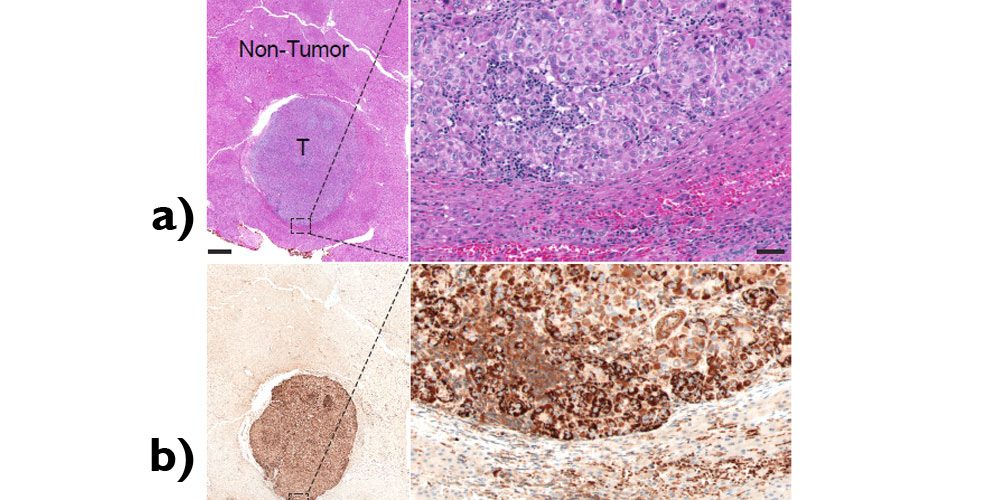
Microscopic images comparing normal liver tissue in (a) to HCC tumour tissue containing PYCR1 enzyme expressed in (b).
SINGAPORE, 7 January 2020 – Researchers at A*STAR’s Singapore Bioimaging Consortium (SBIC) have identified two new possible drug targets for liver cancer treatment. They found that a metabolic pathway for the production of an amino acid called proline, controlled by key genes named PYCR1 and ALDH18A1, was essential for cell proliferation and tumour growth in hepatocellular carcinoma (HCC), the predominant form of liver cancer. This means that finding ways to inhibit the activity of PYCR1 or ALDH18A1 may pave the way towards therapeutic strategies targeting HCC. The research study also provided insights into metabolic changes specific to liver cancer development. The study was a collaboration between A*STAR’s SBIC and Institute of Medical Biology (IMB), the National Cancer Center Singapore (NCCS), and the Pharmaceuticals Division of Bayer1 its findings were published in the Journal of Hepatology in November 2019.
Cancer is a very complex disease. The ability of cancer cells to rewire their metabolism to promote cell growth and proliferation, also known as metabolic reprogramming, plays a pivotal role in their ability to survive and replicate. HCC is the sixth most common cancer worldwide and considered to be the second deadliest cancer for men and sixth for women2. Studies have shown that clinical benefits of available liver cancer treatments are limited3.
The research team led by Prof. Han Weiping, Deputy Director of SBIC and Head of the Laboratory of Metabolic Medicine, aimed to identify targets for developing effective treatments for HCC, by characterising the metabolic shifts in this disease. Tapping on SBIC’s multi-disciplinary capabilities in metabolomics, metabolic imaging, and cancer metabolism, the researchers compared the metabolic enzyme expression profiles of different laboratory models with those from liver tissues of normal and regenerating liver models.
The team demonstrated that the proline biosynthesis pathway, was essential for maintaining a metabolic state that supports the unrestrained growth of HCC tumours. The PYCR1 and ALDH18A1 enzymes involved in proline biosynthesis were found to be highly expressed in HCC tumours, while the depletion of these enzymes suppressed cancer cell proliferation. It was also notable that the team’s analysis of The Cancer Genome Atlas (TCGA) cohort revealed that patients who had low expression levels of PYCR1 or ALDH18A1 had significantly better survival rates. The data from the cohort also indicated that these associations remained significant even after adjusting for different patient and tumour characteristics such as age, tumour stage, tumour grade, radiation therapy, prescription therapy and additional therapies.
These findings collectively suggest that the proline biosynthesis pathway may be a specific and effective target in the treatment of HCC, and the key genes in the pathway PYCR1 and ALDH18A1 may offer a novel therapeutic strategy for liver cancer. Moving forward, the team plans to conduct small molecule screening for drug target validation of PYCR1 and ALDH18A1.
Prof. Han said, “Findings from this study highlight the importance of advancing our understanding of cancer metabolism to identify the main drivers of cancer. By identifying novel factors that can be targeted, our study paves the way for new treatments that can help patients who fail to fully respond to currently-available drugs. As we pursue PYCR1 and ALDH18A1 as targets in liver cancer treatment, we will also test whether these enzymes are also valuable targets for other cancers.”
Prof. Toh Han Chong, Deputy Medical Director (Strategic Partnerships) of NCCS and a co-author of the study said, “These findings create exciting new possibilities for designing treatments against energy pathways that keep liver cancer growing and spreading. This will be a new window to add to drugs that respectively activate an anti-cancer immune system, improve the blood vessel system surrounding cancer and that switch off cancer activating signals.”
Link to online version of the scientific paper:
(https://linkinghub.elsevier.com/retrieve/pii/S0168-8278(19)30667-1)
1 Scientists and researchers who contributed to the studies include Weiping Han, Zhaobing Ding, Russell Ericksen, Qian Yi Lee from SBIC, Nathalie Escande-Beilllard, Abigail Loh, Simon Denil and Bruno Reversade from IMB, Timothy Wai Ho, Pierce Chow and Han Chong Toh from NCCS, and Sylvia Gruenewald, Michael Steckel and Andrea Haegebarth of the Pharmaceuticals Division of Bayer.
2 Source: Journal of Clinical and Translational Hepatology 2018, vol. 6
3 Source: Journal of Clinical and Translational Hepatology 2018, vol. 6
About A*STAR’s Singapore Bioimaging Consortium (SBIC)
The Singapore Bioimaging Consortium (SBIC) under the Agency for Science, Technology and Research (A*STAR), is a leading preclinical bioimaging platform in the world. With a multidisciplinary team of biologists, physiologists, chemists, physicists, electrical/electronic engineers, computer scientists, and clinicians, SBIC investigates human diseases which are major public health issues using molecular physiology and advanced bioimaging tools, in a translational and pivotal mode with the medical community and industrial partners. SBIC also works on strategic bioimaging projects, including the development of novel imaging probes. As a national consortium, SBIC aims to harness existing imaging expertise and capabilities in Singapore, bringing together substantial strengths in the physical sciences and engineering with those in the biomedical and clinical sciences. Through an array of focused collaborations and joint appointments, SBIC fosters and supports the growth of multidisciplinary research activities in the field of bioimaging across local research institutes, universities and hospitals, in order to accelerate the development of biomedical research discoveries. SBIC has a unique capacity to promote rapid transfers of results in animal and human imaging research into the clinical environment, to the immediate benefit of patients. It also ensures the development of financially sound and sustainable contractual research with industrial partners (pharma, food & nutrition, and personal care). SBIC currently operates five joint laboratories with industrial partners under the form of public-private partnerships. For more information about SBIC, please visit www.sbic.a-star.edu.sg
About the Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is Singapore's lead public sector agency that spearheads economic oriented research to advance scientific discovery and develop innovative technology. Through open innovation, we collaborate with our partners in both the public and private sectors to benefit society.
As a Science and Technology Organisation, A*STAR bridges the gap between academia and industry. Our research creates economic growth and jobs for Singapore, and enhances lives by contributing to societal benefits such as improving outcomes in healthcare, urban living, and sustainability.
We play a key role in nurturing and developing a diversity of talent and leaders in our Agency and research entities, the wider research community and industry. A*STAR’s R&D activities span biomedical sciences and physical sciences and engineering, with research entities primarily located in Biopolis and Fusionopolis. For ongoing news, visit www.a-star.edu.sg/.
Was This Article Helpful ?
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
