INNOVATE
Innovate
The Formula Behind Lion TCR's Lifechanging Cancer Therapy
Hepatocellular Carcinoma (HCC), or liver cancer, is the sixth most common cancer worldwidem1 . Yet, treatment options are typically limited to surgery or liver transplantation. Patients with recurrent disease or those who present with late-stage disease have even fewer treatment options, with dismal long-term survival rates.
The Hepatitis B (Hep B) virus is potentially causative for over 50 percent of liver cancer cases worldwide, and as much as 80 percent of cases in Asia. Although vaccination has brought down the incidence rate of Hep B infections, there is still a large unmet medical need to discover effective treatment modalities for current sufferers of Hep B.
To address this unmet medical need, Lion TCR has developed an autologous T-cell immunotherapy against Hep B-associated liver cancer and chronic Hep B. Spun off from A*STAR, this clinical stage biotechnology startup has been working on its proprietary engineered T-cell receptor (TCR) immunotherapy solutions since 2015.
Recently, they have been recognised as the ‘Most Promising Cell & Gene Therapy Startup in Asia’ category at the Asia-Pacific Cell & Gene Therapy Excellence Awards (ACGTEA) 2022.
Lion TCR is moving full speed ahead. Last year, the company received clearance from the U.S. Food and Drug Administration (FDA) for its Investigational New Drug (IND) Application as well as Fast Track Designation for its lead product LioCyx-M004, which is being developed as a potential first-in-class therapy for Hep B-related liver cancer. The FDA Fast Track Designation is reserved for treatments that have demonstrated the potential to solve unmet medical needs for serious medical conditions. Receiving the FDA Fast Track Designation is a significant milestone for Lion TCR, as it expedites the drug approval process to bring such cutting-edge therapies more quickly to patients.
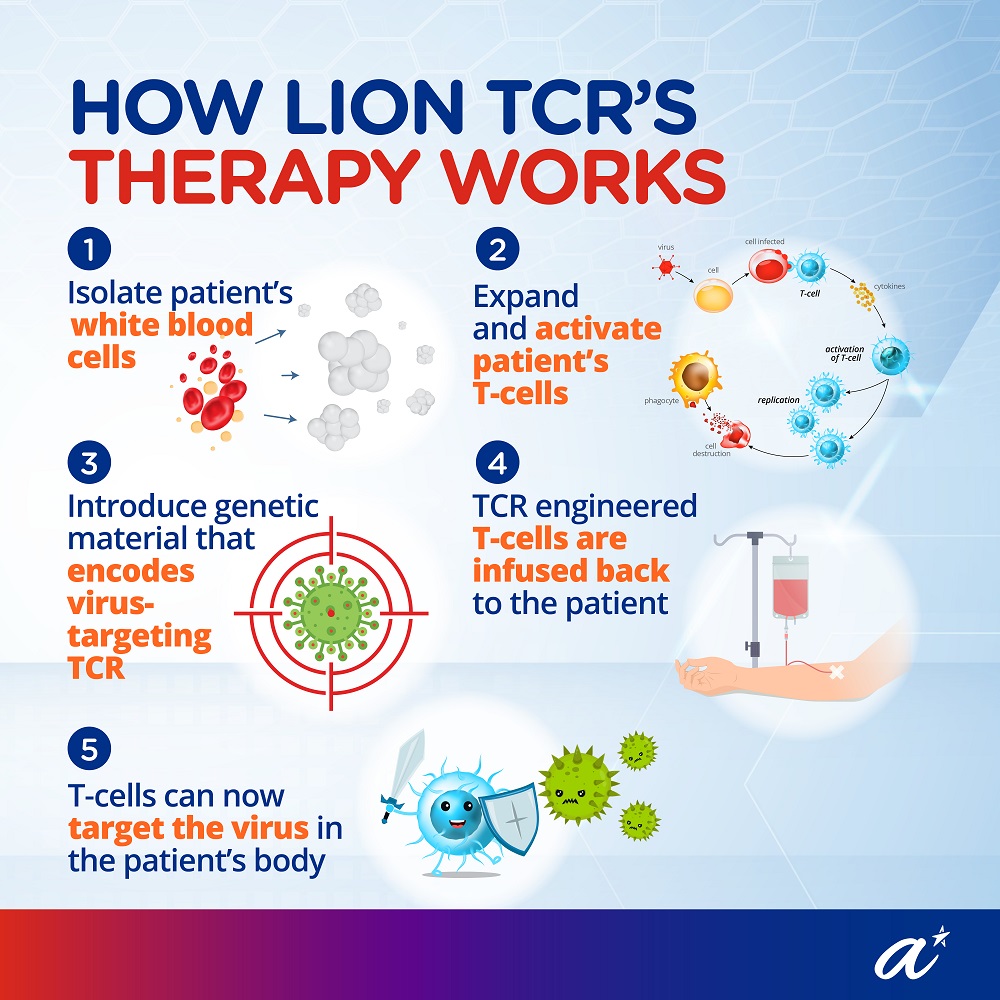
A trial for this TCR-T cell immunotherapy, approved by Health Sciences Authority (HSA), has started recruitment of participants at the Singapore General Hospital (SGH). More information about the trial can be found on the United States’ National Library of Medicine website (ClinicalTrials.gov Identifier: NCT04745403).
To be eligible for participation, the patient must meet the following criteria:
- To be eligible for participation, the patient must meet the following criteria;
- Has a history of chronic hepatitis B;
- Has good and/or compensated liver function;
- Is not suitable for or had failed conventional treatments;
Data gathered from this study will help to:
- Evaluate the safety and efficacy of HBV-specific TCR-T cell immunotherapy
- Understand patients’ immunological changes following HBV-specific TCR-T cell immunotherapy
Those interested in participating in this trial can email clinicaltrials@liontcr.com or contact SGH at (65) 6326 6713.
Lion TCR’s Scientific Founder Professor Antonio Bertoletti and CEO Dr Peng Xiaoming share how the company positioned itself for success.
Identify blue oceans and be first on the scene
In the fiercely competitive biomedical industry, identifying an untapped market and becoming a key solution provider for the market will help companies score a strong competitive advantage.
With limited treatment options for HCC, Lion TCR identified a clear need for new therapies. Lion TCR’s technology is based on research led by Professor Antonio Bertoletti from A*STAR. He initiated the world's first clinical trial using HBV-specific TCR-T cells to treat virus-related cancers. With Prof Bertoletti’s expertise, alongside research partner Duke-NUS Medical School, Lion TCR established itself as the market leader, becoming the first in the world to use HBV-specific TCR-T cell immunotherapy to treat liver cancer.
Technology licensing to shorten time to market
Lion TCR acquired the global exclusive license for A*STAR’s engineered T-cell technology in 2015. With these key patents and exclusive know-how from A*STAR, Lion TCR was able to develop first-in-class therapy for Hep B-related liver cancer in a short time, and move to the clinical stage rapidly.
With strong support from A*STAR and Enterprise Singapore, it subsequently established its R&D and production base in Singapore for the manufacturing of clinical products for use in multi-national clinical studies.
Definitely, technology licensing with A*STAR will provide a jumpstart for companies to gain an advantage in a competitive field. But, the company should have a clear idea on how to use the technology, and ensure it has related expertise to develop the technology into a real pipeline to enter clinical stage.
Professor Antonio Bertoletti, Scientific Founder and Chairman, Lion TCR
Tap on R&D talent to optimise and commercialise the licensed technology
Lion TCR is also building up its intellectual property portfolio and its in-house R&D capabilities to keep its competitive advantage.
This is where R&D talent is crucial. The right expertise will help businesses expand their technical capabilities and build a sustainable innovation pipeline.
Lion TCR found their R&D talent by tapping on A*STAR’s T-Up programme. The T-Up programme seconds A*STAR scientists to companies to help them build up their R&D capabilities and support innovation in product development.
Through the T-Up programme, Dr Sarene Koh, Senior Research Fellow from A*STAR’s SIgN, was seconded to Lion TCR as Director of Technology & Manufacturing. She leads Lion TCR’s product development and process optimisation, taking their solutions from research lab-scale to clinical trial-scale.
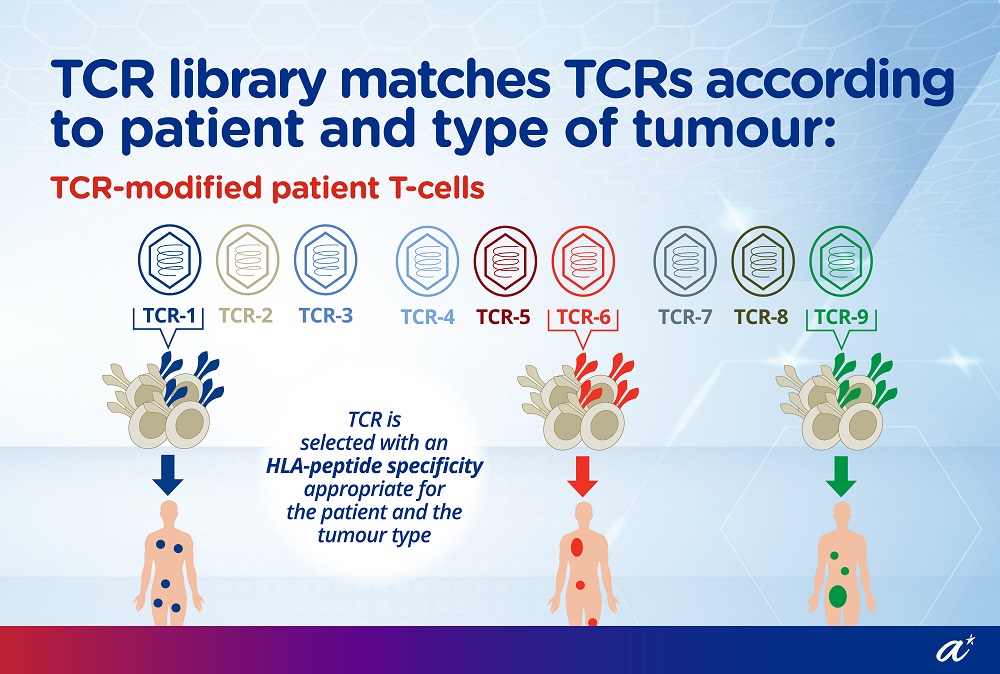
Dr Wai Lu-En, Research Scientist from A*STAR’s SIgN, assumed the role of Director of Research & Development at Lion TCR during the T-Up programme. She built up the company’s HBV TCR library into the largest patented HBV TCR library in the world, covering about 80 percent of the global population. This means that Lion TCR could provide treatment solutions for most patients with HBV related HCC.
The T-Up programme offered Lion TCR access to scientists with valuable knowledge and skills to help build up our technology, especially because we had limited resources. They also helped to link the company closer to A*STAR, which allowed us to deepen the discovery and development of the TCR library, thereby bringing the production of HBV-TCR T-cells from the research lab to clinical studies.
Dr Peng Xiaoming, CEO, Lion TCR
1 Current Treatment Options for Hepatocellular Carcinoma and The Role of Liver Transplantation https://www.sgh.com.sg/news/medical-news-singhealth/hepatocellular-carcinoma-treatment-options
Was the article helpful?
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
