News
Congratulations to IMCB’s latest PhD graduate – Hwee Hui LAU

Thesis Title: Dissecting the role of diabetes-associated PAX4 polymorphisms in modulating pancreatic beta cell development and function
Diabetes is a leading health problem affecting over 537 million individuals worldwide, incurring a huge healthcare burden on society. Populations living in Asia have the highest prevalence of diabetes among all ethnicities and often have a younger age of onset with a lower body mass index (BMI). To develop novel therapeutic strategies, numerous genome-wide studies have been performed across different ancestries to identify genetic variants that can predispose carriers to elevated risks for diabetes. A missense variant within the coding region of the PAX4 gene (rs2233580) is associated with T2D in East Asians. The variant is common in East Asians (MAF 10 %) but rare or absent in other ancestry groups. Carriers of the R192H allele have a dose-dependent earlier age of diabetes-onset and have a lower C-peptide level, suggesting a defect in pancreatic beta cell function.
To elucidate the mechanisms underlying the associations between PAX4variants and the risk of diabetes, our study included detailed clinical and in vitro studies on two distinct PAX4 coding variants. We recruited donors carrying the East Asian-specific R192H variant and a novel protein-truncating variant Y186X identified in a Singapore family for assessment. We demonstrated carriers of the PAX4 R192H variant to have reduced beta cell function, reflected as elevated blood glucose and insufficient insulin secretion. The two carriers of the PAX4 Y186X variants had poor beta cell function as reflected by low disposition index.
Our in silico and in vitro molecular studies predicted that proteins derived from PAX4 R192H and Y186X variants have a loss of function due polymorphism within the DNA binding domain and protein truncation respectively, possibly resulting in reduced beta cell function. In mice, Pax4 is essential for beta cell formation, but neither the role of diabetes-associated variants in PAX4nor PAX4 itself on human beta cell development and/or function are known. To study the consequence of PAX4 variants in human beta cell development, we generated three independent human induced pluripotent stem cell (hiPSC) models. First, a PAX4-knockout hiPSC model was generated using CRISPR-Cas9-mediated genome editing to investigate the role of PAX4 in human pancreatic beta cell development. Second, we generated donor-derived hiPSCs carrying PAX4+/+, PAX4+/R192H, PAX4R192H/R192H and PAX4+/Y186X genotypes to study the consequences of gene variants in beta cell development. Finally, we utilized genecorrected donor-derived hiPSCs to confirm the association of phenotype with the PAX4 variant via a rescue study. Contrary to the observation in rodent models, we found that PAX4 is not required for insulin-expressing beta cell formation from human hiPSCs. We found that, in beta cells derived from hiPSCs that were deficient in PAX4 or carried PAX4 variants exhibited derepression of genes associated with alpha cells. These cells were more likely to be polyhormonal and demonstrated to coexpress GCG+/C-PEP+ in immunostaining assays. These cells also had reduced total insulin content, contributing to decreased functionally. This phenotype was reversed in the donor-derived hiPSC lines through correction of the PAX4 variant allele(s).
Using the human beta cell line EndoC-βH as a model, we demonstrated that PAX4 variant proteins had aberrant transcriptional regulatory activities on INS and GCG gene promoters. The loss of repression of the GCG gene promoter in beta cells possibly explained the coexpression of GCG+ in C-PEP+ endocrine cells carrying PAX4 gene variants. Gene silencing of PAX4 in EndoC-βH1 cells also resulted in elevated GCG expression, reduced total insulin content and impaired glucosestimulated insulin secretion (GSIS) function.
Collectively, we made use of clinical in vivo studies, hiPSC models (including PAX4- knockout, donor-derived hiPSCs and gene-corrected hiPSCs) and mature beta cell line models to sequentially interrogate the role of PAX4 in human beta cell development and function. Our study i) does not support a role of PAX4 variant in causing maturity-onset diabetes of the young (MODY); ii) demonstrated that unlike the mouse, PAX4 is not essential in the differentiation and formation of beta cells in hiPSC models; iii) supports a role of PAX4 deficiency or gene variant in causing the formation of polyhormonal endocrine cells with reduced insulin content and impaired insulin secretion, uncovering a role of human PAX4 in modulating mature beta cell function. Our study therefore facilitates a better understanding of the effects of PAX4 gene variants contributing to the risk of T2D. We conclude that PAX4 R192H (loss of function) and Y186X (haploinsufficiency) variants contribute to the formation of polyhormonal endocrine cells with impaired insulin secretion function, predisposing East Asian carriers to higher risks of developing T2D.
Supervisors: Dr Adrian Teo and Assoc Prof Tan Nguan Soon
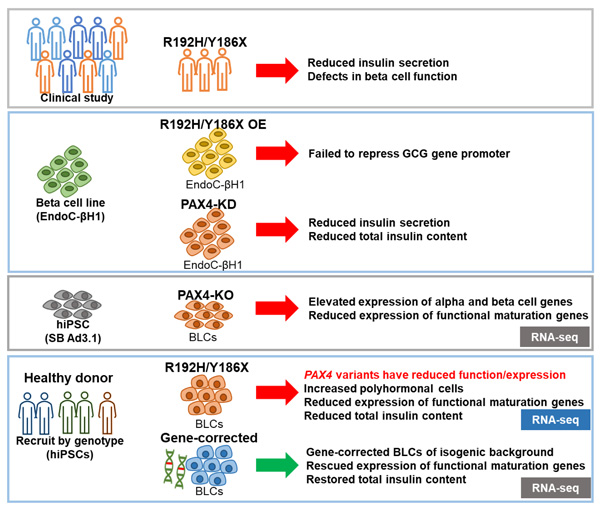
Graphical summary: PAX4 R192H and Y186X contribute to the formation of polyhormonal endocrine cells with impaired insulin secretion function, predisposing carriers to higher risks of T2D. This work contributes to Lau, H. H., et al. (2022). "PAX4 loss of function alters human endocrine cell development and influences diabetes risk." bioRxiv: 2022.2005.2015.491987.
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=c3edc68e_6)