News
New discovery in T-Cell research could lead to improved treatment of solid tumours
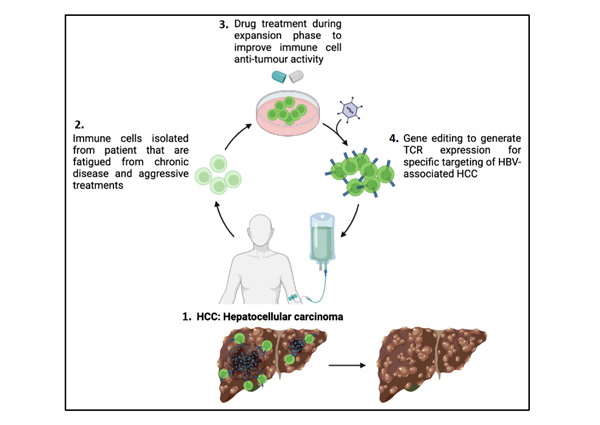
Caption: Cell therapy process for patients with Hepatocellular carcinoma (HCC), a tumour of the liver.
SINGAPORE – A local study led by the Agency for Science, Technology and Research (A*STAR) discovered that by inhibiting the function of two proteins, G9a and GLP, during the cell therapy production process, immune cells could become more effective in combatting cancer. These findings, published in the journal Nature Communications, can help advance the development of innovative therapies that could benefit cancer patients, bringing us closer to more effective targeted treatments for solid tumour cancers.
Solid tumours are a major cause of cancer-related deaths worldwide1. Traditional treatments such as chemotherapy, radiation therapy and surgery are available, but they have differing efficacy against solid tumours, particularly in advanced stages of the cancer2. T-cell therapy has been very successful in targeting liquid tumours such as blood cancers, but the same efficiency has not been observed in solid tumours such as breast, liver or brain cancer.
Engineered T cells are usually introduced into the patient’s bloodstream as part of the treatment. They are in the same environment as liquid tumours, allowing them to locate and target the liquid tumours easily. However, in the case of solid tumours, the engineered T cells face physical and molecular obstacles such as migrating through dense tissue structure inside the body and encountering other cells and molecules that may negatively impact their function3.
Researchers and clinicians from A*STAR’s Institute of Molecular and Cell Biology (IMCB) and Singapore Immunology Network (SIgN), and Duke-NUS Medical School collaborated to explore innovative approaches to improve the efficiency of T cells, the immune cells responsible for recognising and eliminating cancer cells.
The research team, led by Dr Andrea Pavesi, Senior Scientist at A*STAR’s IMCB, conducted a comprehensive analysis of epigenetic drugs that can affect the efficacy of the engineered T cells in increasing anti-tumour activity. The team used 2D and novel 3D assays that mimicked the physical environment that T cells would encounter to find and target the cancer cells in the human body. A drug was administered to the immune cells during the cell expansion process of cell therapy performed in the lab, which targeted the G9a and GLP proteins. The drug was subsequently washed away before the engineered immune cells were re-introduced into the patient’s body, thus eliminating side-effects from the drug. The findings showed that the drug helped to increase the anti-tumour function of the engineered immune cells – it increased the production of granzymes, proteins that help to locate and eliminate target tumour cells.
Tapping on the immune cell profiling capabilities of Dr Giulia Adriani, Principal Scientist at A*STAR’s SIgN, and patient samples from Duke-NUS, the study’s findings were validated using well-established cell-lines and patient-derived immune cells to confirm the efficacy of blocking G9a and GLP activity in improving the efficiency of T-cell therapy. The results showed that the drug enhanced the anti-tumour function of engineered immune cells.
This would mean better patient outcomes such as improved survival rates and quality of life. It also has broad implications for all cell therapies targeting solid tumours. Patients with a weak immune system, who usually require immune cells from healthy donors for cell therapy treatment, may also benefit from treatments using their own immune cells. This reduces the chances of the patient’s body rejecting the cells, as well as implications from using incompatible cells. The drug used to block G9a and GLP activity also holds significant potential for further development, presenting itself as an attractive therapeutic option for cancer treatments.
Dr Andrea Pavesi, Senior Scientist at A*STAR’s IMCB and lead author of the study, said, “The approach of improving the individual anti-tumour activity of each immune cell can address many limitations in T-cell therapy and enhance treatment efficacy.
Our discovery will advance the development of effective therapeutics for solid tumour cancers and help improve lives.”
Professor Antonio Bertoletti, from Duke-NUS’ Emerging Infectious Diseases Programme, said, “There is a high demand for the production of suitable T cells for adoptive T-cell therapy, a type of cell therapy where engineered T cells are administered to patients to fight diseases such as cancer. This discovery could help improve cell therapies that use both patient-derived and donor-derived immune cells, benefitting a variety of patients. We hope to work towards successful clinical trials and bring this method to market to improve patient outcomes.”
1 World Cancer Research Fund International
2 The Emerging Landscape of Immune Cell Therapies, Cell, Volume 181, Issue 1, April 2020
3 Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead, Journal for
ImmunoTherapy of Cancer, Volume 9, Issue 7, July 2021
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=c3edc68e_6)