INNOVATE
Interviews
Story of dialysis device startup Advent Access
Published on 14 Feb 2019 | By Claudia Chong | Source: Business Times © Singapore Press Holdings Limited. Permission required for reproduction.
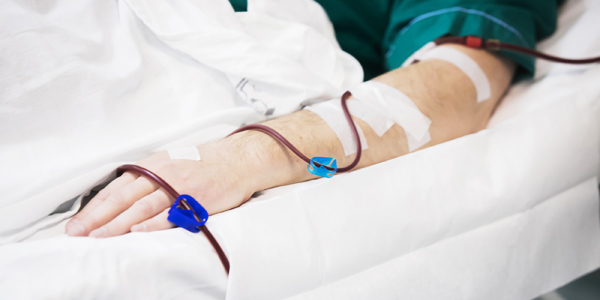
KIDNEY-failure patients undergoing hemodialysis suffer numerous needle insertions every week- with each prick running the risk of pain- ineffectiveness and wearing out the target vein.

Peh Ruey Feng- founder of Advent Access- says being able to handle needle insertion by themselves is an important freedom for kidney-failure patients undergoing dialysis. SPH
Medical device startup Advent Access is going after that literal pain point- with the goal of ultimately making needling so easy that patients can do it by themselves. The company has a core product- av-Guardian- on the verge of obtaining CE market approval- and a recently acquired already-commercialised solution that could help to jumpstart growth.
“(Allowing patients to needle themselves) might sound like a good-to-have- but the significance of that is that it is the biggest bottleneck in freeing the patient from the nurse and the infrastructure-” said Advent Access founder and chief executive Peh Ruey Feng.
Mr Peh spun off Advent Access in late 2015 from Singapore’s Agency for Science- Technology and Research (A*Star). Accuron MedTech- which became directly owned by Singapore government-owned investment firm Temasek Holdings in January- was an early backer that led the startup’s S$2.6 million pre-Series A financing round in 2017. Angel investors include Lu Yoh-Chie- founder and ex-CEO of SGX-listed Biosensors International- and Steven Fang- founder and ex-CEO of SGX-listed Cordlife.
av-Guardian- the startup’s core product- is a device about one and a half times the size of a fingernail that is implanted underneath the skin in the arm- just above a vein.
The device guides a needle precisely to a vein that is used for hemodialysis treatment. The idea is to engineer a scar-tissue track that links from underneath the skin to the vein- which improves access and reduces the wear and tear on the vein. This can help to reduce the number of surgeries and hospitalisations that a patient might need to go through- and eventually patients could potentially undergo dialysis with a blunt needle almost painlessly- akin to “putting on your earring”- said Mr Peh.
A multi-centre study completed in 2017 at the Singapore General Hospital and the National University Hospital showed that the device is safe- achieves clinical performance and can be considered a significant support for chronic dialysis patients.
A second study on self-cannulation – the insertion of the treatment tube – and self-hemodialysis is ongoing in New Zealand and targeted to complete in the middle of this year.
Advent Access is currently awaiting CE mark approval for av-Guardian- which will allow it to be sold in Europe and certain parts of Asia. After that milestone- the firm will seek Food and Drug Administration approval in the US- and begin commercialisation in New Zealand- Australia and selected parts of Europe.
Another round of fundraising is likely after CE mark approval- Mr Peh said. He declined to disclose what the funds will be used for- but said there are already some interested parties.
While av-Guardian navigates the regulatory process- Advent Access in 2018 acquired the global manufacturing and commercialisation rights to the Vwing Vascular Needle Guide product line and other key assets from US firm Vital Access for an undisclosed sum.
Like av-Guardian- Vwing is an implant that provides a target for needle insertion- except that it clings onto the arteriovenous fistula (AV fistula)- a surgically created passageway between an artery and a vein used for dialysis. Vwing is targeted at more severe cases- however- most of which involve obese patients with very deep fistulas.
Vwing is already commercialised- and has been adopted by more than 70 clinical sites in the United States and successfully used in more than 150-000 patient dialysis sessions. But Advent Access will have to put in some work to see meaningful revenue from the product because Vital Access had stopped selling Vwing due to financial challenges when Advent Access bought the rights.
Mr Peh is taking a cautious approach to restarting Vwing sales and the temptation of commercialising av-Guardian too rapidly.
“We don’t want to hastily jump back into the market-” he said about Vwing. “My wish is to actually go back to really listen to the customers again in that geography and understand what they like about our product and what they feel could still be improved. I do actually have a vision that we could introduce the product back into the market with a different mentality.”
Vwing will ultimately give Advent Access a fuller range of solutions to address vascular access- and a leg up among potential customers as it moves toward commercialising av-Guardian- Mr Peh said.
“The acquisition is really a strategic business move for us that allows us to...gain an early access to the US market- which is the world’s largest hemodialysis market at US$26.7 billion- in terms of spending (in 2015)-” he said.
It is clear that Advent Access has its sights set on the global dialysis market.
“We’re operating in a space where kidney failure is driven by two pandemics – diabetes and hypertension. This is a patient population that is set to double in the next ten years- worldwide-” Mr Peh said.
He highlighted that kidney failure is already consuming about 7 per cent of the budget for the US Centres for Medicare and Medicaid Services- even though the reimbursed patients form less than 1 per cent of the total patient population.
“It’s a market that is growing very rapidly.”
Advent Access at a glance
- Founder and CEO: Peh Ruey Feng. Founding faculty of Singapore-Stanford Biodesign- a joint programme between A*Star- the Economic Development Board and Stanford University that trains medical device innovators.
- Business: Medical device for hemodialysis treatment
- HQ: Singapore
- Backers: Accuron MedTech- A*Star- Seeds Capital (pre-Series A)
- Funding rounds: S$2.6 million (pre-Series A- 2017); undisclosed (Seed- Q3 2015)
Was the article helpful?
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
