Pioneering Immunotherapy For Liver Cancer: A*STAR's Spin-Off Lion Tcr
Liver cancer remains one of the deadliest cancers globally, with limited treatment options and poor outcomes for advanced-stage patients. Recognising this unmet need, Lion TCR, an A*STAR spin off, developed a T-cell receptor (TCR) immunotherapy targeting Hepatitis B-related liver cancer — leveraging deep immunological research and public-private collaboration to bring a potentially first-in-class therapy to clinical trial. With support from A*STAR’s T-Up programme, the company advanced swiftly from bench to bedside, gaining regulatory clearance from the U.S. Food and Drug Administration (FDA).
Jump to Section: The Challenge | Our Innovation | The Impact

The Challenge
Hepatocellular carcinoma (HCC), the most common form of primary liver cancer1, is especially prevalent in Asia, where Hepatitis B virus (HBV) infection accounts for up to 80% of the cases.
- Current treatment gap: Treatment options are typically limited to surgery or liver transplantation. Those with recurrent or late-stage disease face dismal long-term survival rates.
- Large unmet clinical need: Chronic HBV infection persists globally despite vaccination effort and there is still a large unmet medical need to discover effective treatment modalities for current sufferers of Hep B.
Our Innovation
Lion TCR, a clinical-stage biotech spun off from A*STAR, is the first in the world to use HBV-specific TCR-T cell immunotherapy to treat liver cancer.
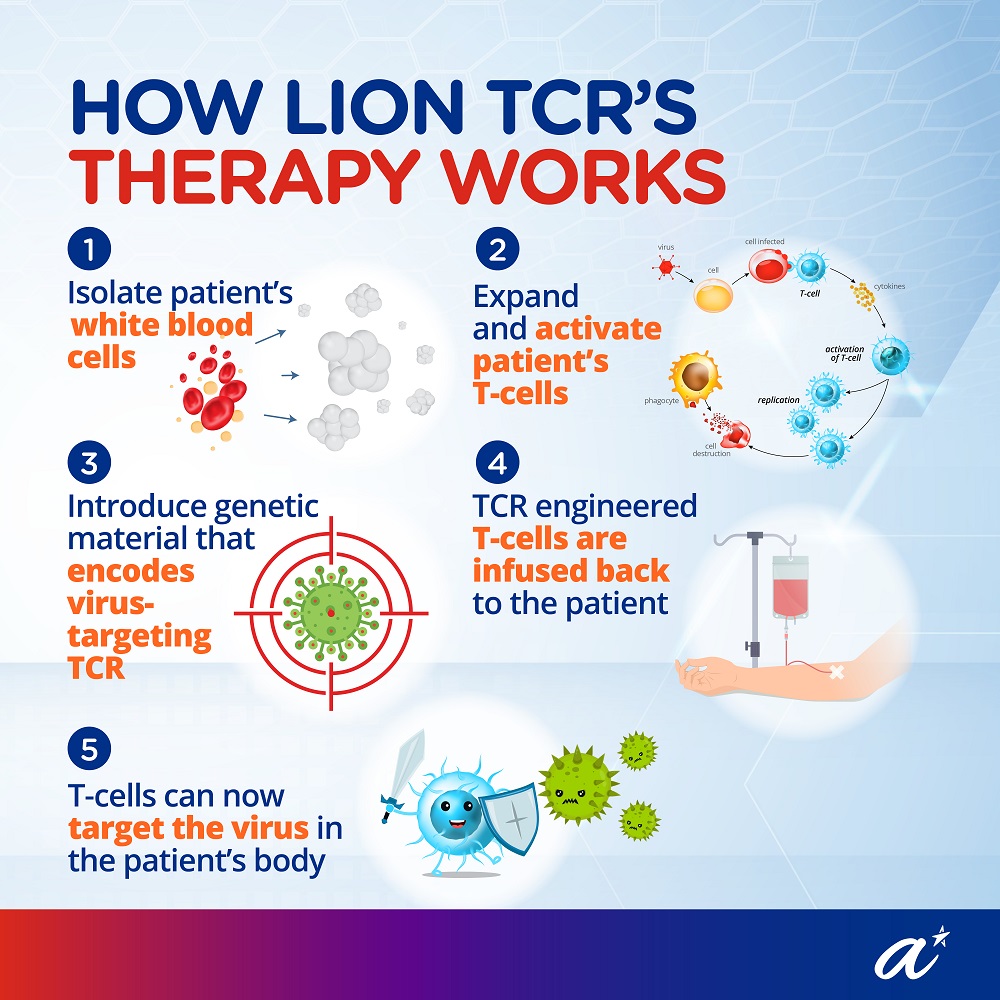
- Scientific foundation: Founded on research by Prof Antonio Bertoletti at A*STAR, Lion TCR initiated the world’s first trial using HBV-specific TCR-T cells.
- Strategic licensing: In 2015, Lion TCR secured exclusive rights to A*STAR’s T-cell receptor technology, enabling rapid clinical translation and IP protection.
- Talent infusion through T-Up: A*STAR’s T-Up programme embedded R&D talent into the company:
- Dr Sarene Koh (A*STAR SIgN) led the transition from lab-scale development to clinical manufacturing.
- Dr Wai Lu-En (A*STAR SIgN) built the world’s largest patented HBV TCR library, covering ~80% of the global population.
The T-Up programme offered Lion TCR access to scientists with valuable knowledge and skills to help build up our technology, especially because we had limited resources. They also helped to link the company closer to A*STAR, which allowed us to deepen the discovery and development of the TCR library, thereby bringing the production of HBV-TCR T-cells from the research lab to clinical studies.
-Dr Peng Xiaoming, CEO, Lion TCR
- Global acceleration: With FDA clearance for Investigational New Drug (IND) application and Fast Track Designation for its lead product LioCyx-M004, Lion TCR became the first Singapore biotech to receive such status for a T-cell therapy.
The Impact
Lion TCR's work demonstrates how A*STAR’s translational research ecosystem can enable global health breakthroughs and economic competitiveness.
- Pioneered a novel, personalised treatment for HBV-related liver cancer, now in clinical trials at Singapore General Hospital.
- Positioned Singapore as a regional hub for cell and gene therapy innovation.
- Expanded access to life-extending therapies for previously untreatable patients.
1 Current Treatment Options for Hepatocellular Carcinoma and The Role of Liver Transplantation https://www.sgh.com.sg/news/medical-news-singhealth/hepatocellular-carcinoma-treatment-options
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM