mRNA BioFoundry
Manufacturing Nucleic Acids for the Future
The mRNA BioFoundry focuses on (1) process development, optimisation and innovation for mRNA manufacturing; and (2) development of analytical assays for drug substance and drug product release.
We develop and implement cGMP-compatible processes to enable seamless transition from research to manufacturing in a regulated environment. Our work supports the clinical translation of mRNA-based assets by providing access to preclinical-grade formulated mRNA. Additionally, we drive automation and integration of advanced manufacturing technologies and analytics for end-to-end production. As part of our commitment to pandemic readiness, we are establishing GMP-ready cleanroom facilities incorporating these innovative processes.
Focus Areas
- Establishment of scalable, GMP-compatible workflows to enable transition from research to clinical manufacturing
- Integration of technologies for efficient end-to-end production
- Innovation in nucleic acid production processes and analytics
- Evaluation of novel technologies and platforms for mRNA manufacturing
Our Role in Ecosystem
Manufacturing Capabilities
Bespoke Solutions
Customisable workflows for different process development and production scales will be set up based on the requirements of specific mRNA products.Enzymatic, Cell-free DNA Amplification
Faster process than fermentation and fewer issues with sequence complexities.Production Innovation
Manufacturing process development for other nucleic acid modalities beyond mRNA, e.g. saRNA, circular RNA etc.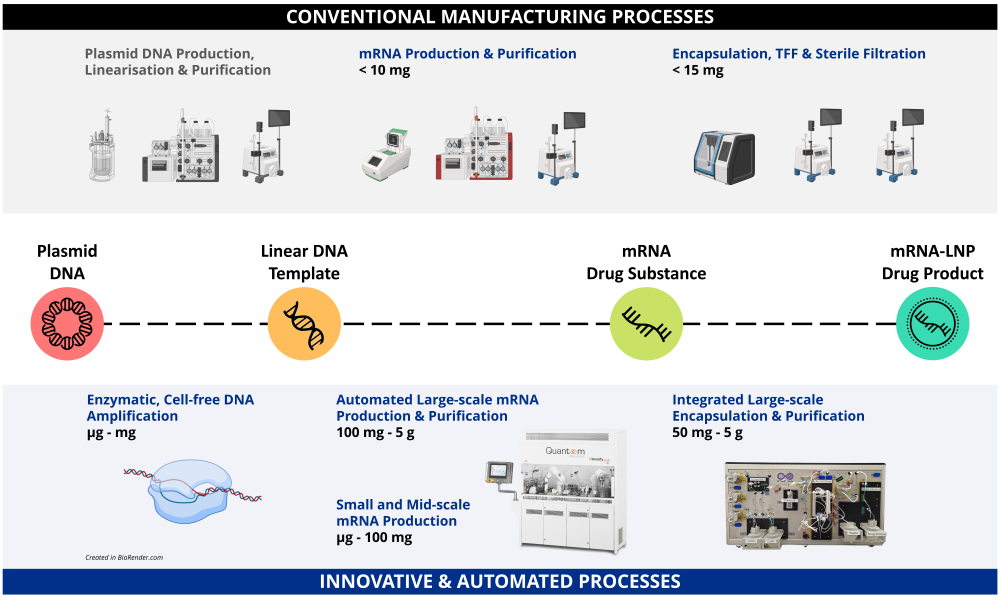
Analytical Suite
Validated Critical Quality Attribute (CQA) Assays
Aligned with regulatory requirements and/or US Pharmacopoeia (USP) guidelines for in-process and final release.Fit-for-Purpose Analytics
Tailored to different needs (e.g. research or preclinical grade).Analytical Method Development
Advancing analytical methodologies for the detection of emerging CQAs, in close partnership with AS&T Metabolomics group.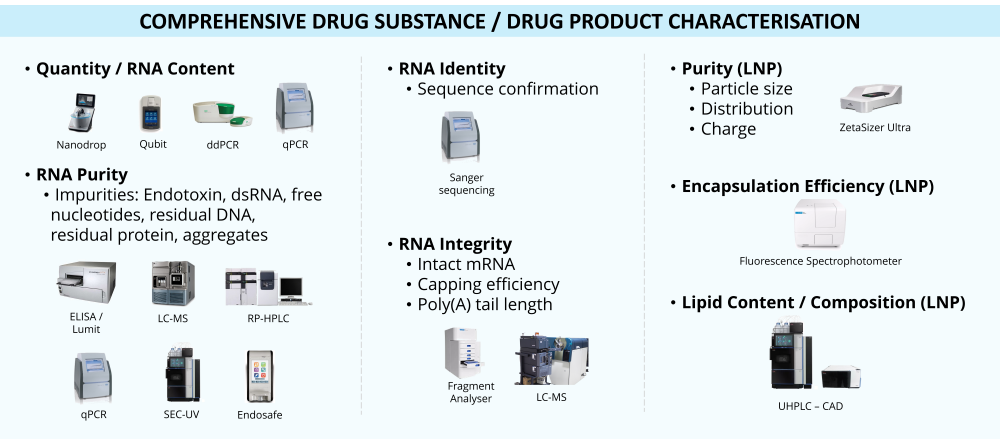
The Team
.png?sfvrsn=51564096_0)
.png?sfvrsn=9bef6a4f_0)
.png?sfvrsn=3264e39b_0)
Ng Sze Wai
Lead Research Officer I
.png?sfvrsn=f66dd463_0)
Chen Huishan
Senior Research Officer I
.png?sfvrsn=297fc88b_0)
Sim Lyn Chiin
Senior Research Officer I
Our Track Record
- Awarded collaborative grant for development of influenza vaccines
- Validated production batches from 1 mg to 1 g
- More than 20 mRNA-LNPs produced for academic and industrial partners
Featured Publications
- Jing Liang, Huibin Zhang, Yee Ling Tan, Huimin Zhao and Ee Lui Ang (2022) Directed evolution of replication-competent double-stranded DNA bacteriophage toward new host specificity. ACS Synthetic Biology 11(2):634-643
- Shuhui Lim, Regina Khoo, Yu-Chi Juang, Pooja Gopal, Huibin Zhang, Constance Yeo, Khong Ming Peh, Jinkai Teo, Simon Ng, Brian Henry and Anthony W. Partridge (2020) Exquisitely specific anti-KRAS biodegraders inform on the cellular prevalence of nucleotide-loaded states. ACS Central Science 7(2): 274-291
- Sidika Tapsin, Miao Sun, Yang Shen, Huibin Zhang, Xin Ni Lim, Teodorus Theo Susanto, Siwy Ling Yang, Gui Sheng Zeng, Jasmine Lee, Alexander Lezhava, Ee Lui Ang, Lian Hui Zhang, Yue Wang, Huimin Zhao, Niranjan Nagarajan and Yue Wan (2018) Genome-wide identification of natural RNA aptamers in prokaryotes and eukaryotes. Nature Communications 9: 1289
- Huibin Zhang, Teodorus T. Susanto, Yue Wan, and Swaine L. Chen (2016) Comprehensive mutagenesis of the fimS promoter regulatory switch reveals novel regulation of type 1 pili in uropathogenic Escherichia coli. Proceedings of the National Academy of Sciences 113(15): 4182-4187
- Huibin Zhang, Karen L Artiles and Andrew Z Fire (2015) Functional relevance of “seed” and “non-seed” sequences in microRNA-mediated promotion of C. elegans developmental progression. RNA 21(11): 1980-1992
Connect with Us
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)