Process Accelerator for Cell Therapy Manufacturing (PACTMAN)
De-Risk and Accelerate cGMP Manufacturing of Cell Therapy
PACTMAN’s role in the cell therapy (CT) ecosystem is to derisk and accelerate transition of CT assets from R&D towards cGMP manufacturing for clinical deployment. We work closely with CT asset developers and cGMP manufacturing organizations to provide pre-GMP process and analytical development support. We ensure that critical unit operations are optimized and end-to-end manufacturing and analytical workflows are well established to ensure a seamless transition towards cGMP manufacturing.
Focus Areas
- Process transfer gap analysis for CT manufacturing workflow.
- Pre-GMP optimization of critical process parameters for immune cell therapy and regenerative cell therapy manufacturing.
- Fit-for-purpose development of QC analytical release or characterization assays for CT pipelines.
- Development of closed system bioreactor workflows for autologous and allogenic CT pipelines.
- Evaluation of novel manufacturing and analytical platforms and workflows for cell therapy.
Our Capabilities
Process Transfer Gap Analysis
Perform gap analysis and identify studies needed to optimize critical unit operations as well as ancillary material evaluation for cGMP manufacturing.Pre-GMP Process Development
Scale-down process development to optimize critical process parameters and define parameter ranges using quality by design approach such as DoE methodology. Perform end-to-end manufacturing at-scale to demonstrate reproducibility of manufacturing workflow to achieve quality target product profile.Fit-for-purpose Analytical Development
Support with fit-for-purpose analytical method development for QC release assays and process characterization assays based on ICHQ2 requirements.Chemistry, Manufacturing and Controls
Provide supporting CMC data for investigational new drug (IND) or clinical trial application (CTA) filling. This involves: understanding mechanism of action or chemistry of your cell therapy product through purity and potency assessment, specification of manufacturing process parameters and ranges to ensure consistent and safe manufacturing, and process controls to ensure final products meet the quality requirements in terms of identity, purity, potency, safety and stability.Technology transfer, drafting of SOP and batch records for cGMP Manufacturing
Support technology transfer of CT pipelines and training of manufacturing and QC staff on the optimized manufacturing workflow and analytic methods developed by our team. Support with drafting out SOPs and manufacturing batch record forms to be transferred over to cGMP manufacturing and QC teams.
Our Technologies
Manufacturing Workflow Development
Working with CT asset developers and ACTRIS as well as industry solution providers, PACTMAN aims to develop optimized and cGMP-compatible end-to-end manufacturing workflows for multiple CT assets such as CAR-T, TCR-T, gamma-delta T, NK, tumor-infiltrating lymphocytes as well as regenerative medicine assets such as mesenchymal stem cells and induced pluripotent stem cells differentiated pipelines.Analytical Method Development
PACTMAN aims to develop standardized and phase-appropriate qualified analytical methods to support release testing of manufactured CT products as well as for in-depth process characterization. These include assays for determination of identity and purity (Flow cytometry phenotype, transduction efficiency), potency (cell-killing toxicity assay, cytokine secretion), safety (proliferation assay to assess irradiation efficiency, reprogramming vector clearance, vector copy number determination) and quality (cell viability).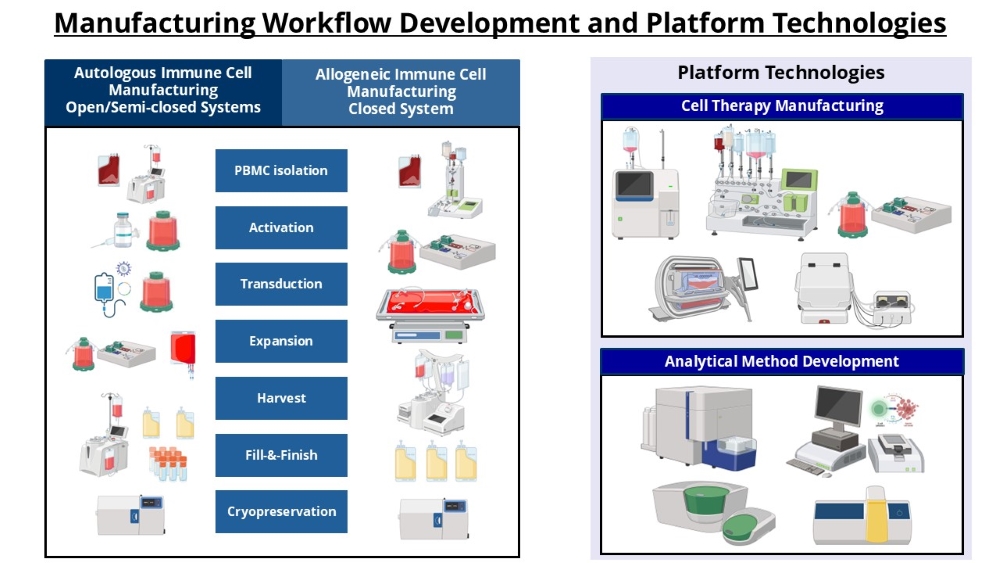
The Team
.jpg?sfvrsn=bf5d00a1_0)
.jpg?sfvrsn=dcd3dfcf_0)
Our Track Record
Featured Publications
- Viknesvaran Selvarajan, Denise Bei Lin Teo, Chaw-Chiea Chang, Yuen Ling Ng, Nge Cheong, Jaichandran Sivalingam, Soo Hean Gary Khoo, Adison Wong and Bernard Liat Wen Loo (2024) Piloting a scale-up platform for high-quality human T-cells production. Frontiers in Cell and Developmental Biology 12: 1427171
- Jaichandran Sivalingam, Yu SuE, Zhong Ri Lim, Alan T.L. Lam, Alison P. Lee, Hsueh Lee Lim, Hong Yu Chen, Hong Kee Tan, Tushar Warrier, Jing Wen Hang, Nazmi B. Nazir, Andy H.M. Tan, Laurent Renia, Yuin Han Loh, Shaul Reuveny, Benoit Malleret and Steve K.W. Oh (2021) A Scalable Suspension Platform for Generating High-Density Cultures of Universal Red Blood Cells from Human Induced Pluripotent Stem Cells. Stem Cell Reports 16(1):182-197
- Jaichandran Sivalingam and Steve Kah-Weng Oh (2019) Expert Insight: Bioprocessing and biological challenges of large-scale red blood cell production from pluripotent stem cells: Important factors to consider. Cell & Gene Therapy Insights 5(3): 483-504
- Sze Sing Lee, Jaichandran Sivalingam, Ajit J Nirmal, Wai Har Ng, Irene Kee, In Chin Song, Chin Yong Kiong, Kristoffer A Gales, Frederic Chua, Edgar M Pena, Bryan E Ogden and Oi Lian Kon (2018) Durable engraftment of genetically modified FVIII-secreting autologous bone marrow stromal cells in the intramedullary microenvironment. Journal of Cellular and Molecular Medicine 22(7): 3698-3702
- Jaichandran Sivalingam, Dimitar Kenanov, Hao Han, Ajit Johnson Nirmal, Wai Har Ng, Sze Sing Lee, Jeyakumar Masilamani, Toan Thang Phan, Sebastian Maurer-Stroh and Oi Lian Kon (2016) Multidimensional Genome-wide Analyses Show Accurate FVIII Integration by ZFN in Primary Human Cells. Molecular Therapy 24(3): 607-619
Connect with Us
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)
.jpg?sfvrsn=7942b38f_0)
.png?sfvrsn=93ae0c6d_0)
.jpg?sfvrsn=ffba3294_0)