COVID-19 Stories

FEB '20
May '20


JUN '20
Resolute 2.0
JUN' 20
RAVE

miRNA
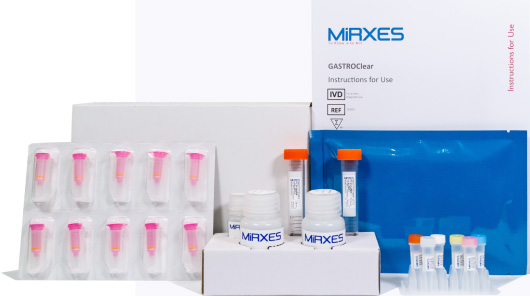
GASTROClear
GASTROClear is the world’s first approved microRNA blood test for early detection of gastric cancer offered in ClinLab. It was co-developed with MiRXES, Singapore Gastric Cancer Consortium (SGCC), National University Hospital (NUH), and Tan Tock Seng Hospital (TTSH).
- High performance test which detected 87.5% of stage I gastric cancers and 89.5% of stage II gastric cancers in clinical validation.
- Minimally invasive procedure which requires only 5 millilitres of patient’s blood.
- An actionable diagnostic test for cancer screening which gives a quantitative risk score and classifies each patient into a risk group.
Digital Health
Respiree Monitor
Respiree monitor is a wearable wireless device that monitors COVID-19 patients by predicting worsening conditions. It was co-developed with Respiree and Singapore Bioimaging Consortium (SBIC).
- Allows for comfortable wearing without sticking to skin.
- Measures vital signs and trends to provide a risk score, thus predicting health deterioration.

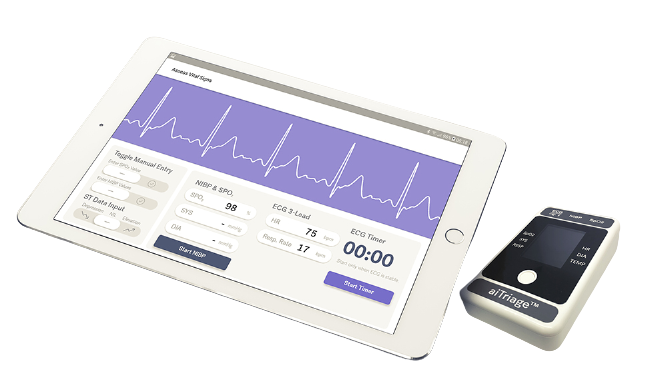
aiTRIAGE™
aiTRIAGE™ is a SaMD using (clinical-based) machine learning algorithm to assist clinicians in quickly and accurately triaging patients at risk of Major Adverse Cardiac Event (MACE). It is currently in development with TIIM Healthcare (SGH and NHIC).
abTES COVID-19
- Co-developed with Genome Institute of Singapore (GIS), this kit is based on loop mediated isothermal amplification (LAMP) technology, which enables rapid amplification and detection of COVID-19. Following licensing to AITBiotech, the abTES COVID-19 has been made commercially available in the market, as well as becoming CE IVD certified.
INSPECTA
- The INSPECTA is an Automated Reverse Transcription Polymerase Chain Reaction (RT-PCR) Curve Analysis and Test Interpretation system to be used with the RESOLUTE 2.0 Direct PCR Test Kit for SARS-CoV-2 Detection. It has received HSA approval as a Class A SaMD, and has been validated with both Bio-Rad’s CFX qPCR and Applied Biosystems’ QuantStudtio 5 Systems. The INSPECTA has since been made commercially available under a licensing agreement with Alliance Biomed, and has been deployed at a local commercial laboratory.
REDAPT
- The REDAPT is a sample collection kit with proprietary chemistry, enabling deep throat saliva (DTS) specimen collection for RESOLUTE 2.0. It is the only test kit approved for use with the RESOLUTE 2.0 test kit.
TISSUE500
- The TISSUE500 is a pan-cancer assay, enabling Asian-centric molecular characterization of tumours based on Next-Generation Sequencing (NGS) technology. Commercially launched in 2021, the Tissue500 was showcased in both local and international conferences, such as the Frontiers in Cancer Science Conference 2021, and the United States & Canadian Academy of Pathology (USCAP) 2022 Annual Meeting. The test is offered as a Laboratory Developed Test (LDT) by Lucence, with services provided by Lucence's CAP lab in SG, catering to local and regional hospitals.
FxMammo
- The FxMammo is an AI-assisted algorithm that screens for breast cancer automatically and accurately from digital mammography images. It is one of six innovations (of 256) to receive an award at the Healthcare Innomatch 2022. Using the FxMammo, false positives have been demonstrated to be reduced from 9% to 2%.
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
