News and Events
Singapore-developed H5 Avian Influenza Virus (AIV) Test Kit Commercialised and Licensed for Regional Distribution
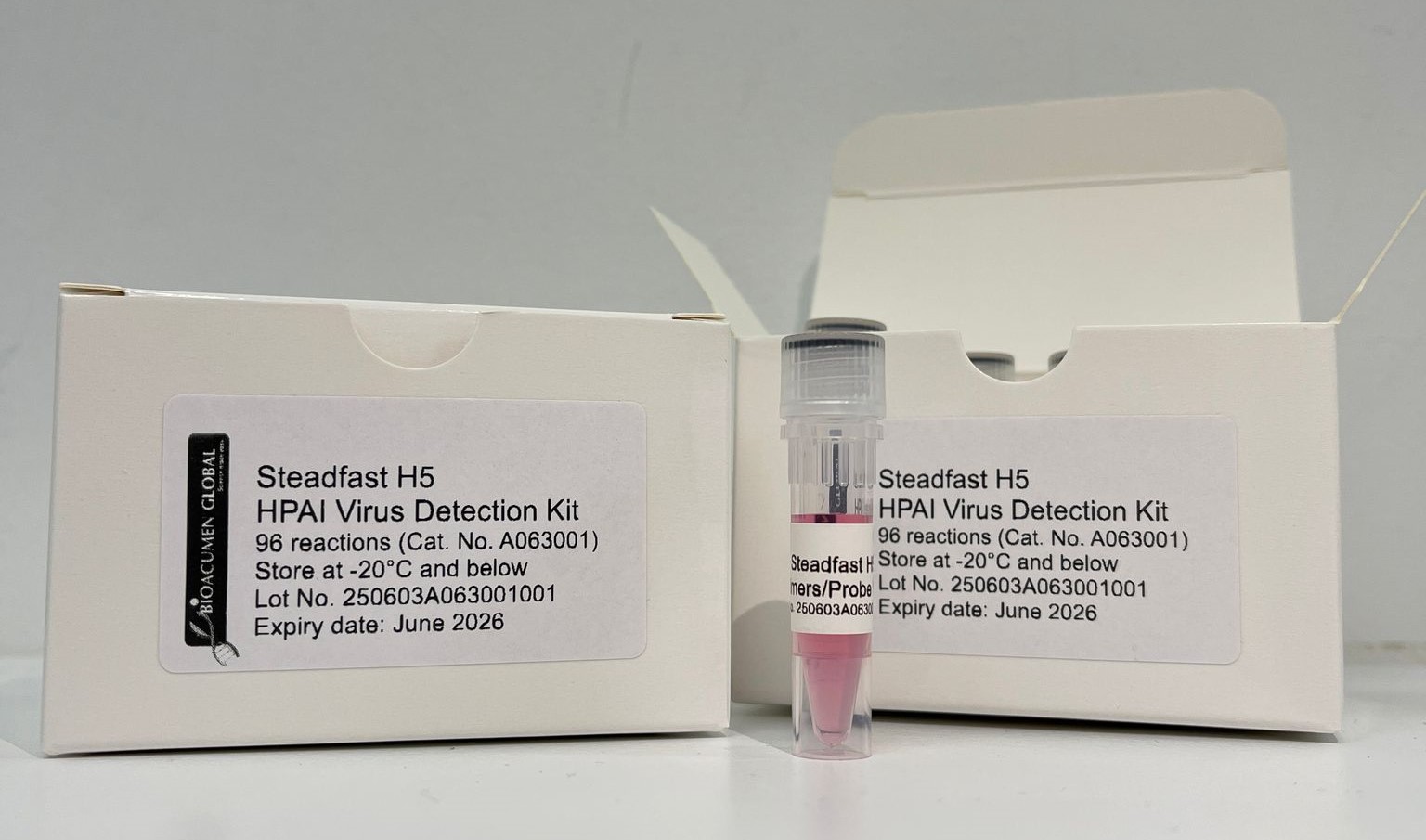
Photo: Singapore-developed H5 Avian Influenza Virus (AIV) Test Kit Commercialised and Licensed for Regional Distribution
Singapore – Steadfast H5-HPAI, a Singapore-developed diagnostic kit for detecting the highly pathogenic H5 Avian Influenza Virus (AIV), has reached a significant milestone with its successful commercialisation and licensing for laboratory use.
The assay was developed in November last year, through an international collaboration between Diagnostics Development Hub (DxD Hub), a national platform hosted by the Agency for Science, Technology and Research (A*STAR), Singapore, National Institute for Environmental Studies (NIES), Japan, and A*STAR Bioinformatics Institute (A*STAR BII). It has since been translated into a market-ready product.
Steadfast is a high-performance diagnostic kit designed for use in centralised laboratories, enabling rapid and accurate detection of highly pathogenic avian influenza viruses (AIV) within three hours. Its high sensitivity and specificity make it a critical tool for early outbreak detection and timely biosecurity response. Central laboratory settings—equipped with advanced instrumentation and rigorous quality controls—provide an ideal environment for Steadfast to deliver consistent, reliable results at scale. While field-based methods play an important role in rapid, on-site testing, laboratory-based diagnostics like Steadfast support large-volume testing and seamless data integration into national surveillance systems. This enables timely alerts, coordinated action, and stronger protection of poultry and public health across the region.
The assay has been non-exclusively licensed to Singapore-based biotech startup BioAcumen Global Pte Ltd, which will distribute the kits to national surveillance centres, veterinary clinics, hospitals and private laboratories across Southeast Asia by December 2025.
“Our goal is to make advanced diagnostics more accessible and responsive to real-world needs,” said Jimmy Toh, Director and Chief Scientist of BioAcumen Global Pte Ltd. “This assay enables poultry farmers and health authorities to detect outbreaks early and take swift action to contain them.”
Dr Weng Ruifen, Chief Executive Officer of DxD Hub, remarked: “We are very excited to partner with BioAcumen to translate our diagnostic innovations into real‑world solutions. By combining their commercialisation expertise with our scientific and diagnostics productisation know‑how, we can bring these diagnostics to end-users across the region more swiftly. This collaboration also nurtures vital home‑grown expertise to bolster regional preparedness against emerging infectious diseases.”
The successful commercialisation of Steadfast, underscores the power of public-private collaboration in accelerating healthcare innovation. With regional rollout underway, the technology is set to play a vital role in improving avian flu surveillance and response throughout Asia.
– End –
About DxD Hub
The Diagnostics Development Hub is a national platform hosted by the Agency for Science, Technology and Research (A*STAR). DxD Hub aims to accelerate the transformation of innovations into clinically validated diagnostic devices that are ready for market adoption. Through impactful products, empowering local enterprises and anchoring global companies in Singapore, DxD Hub contributes to the development of an effective diagnostic devices’ ecosystem in Singapore. For ongoing news, visit www.a-star.edu.sg/dxdhub
About BioAcumen Global Pte Ltd
BioAcumen Global Pte Ltd is a Singapore-based biotechnology company specializing in contract manufacturing, diagnostic solutions, and veterinary testing services. With ISO 13485 certification, we uphold the highest standards in quality and regulatory compliance for the production of medical devices and in-vitro diagnostic reagents. Our core capabilities include small- to mid-scale manufacturing, lyophilisation (freeze-drying) of diagnostic products, development of in-house PCR kits and customized formulation services to support research institutes, healthcare providers, and biotech companies. We also provide molecular testing services for companion animals, contributing to enhanced veterinary care. Beyond diagnostics, BioAcumen distributes a range of life science products, operates an educational hub to support scientific learning, and offers breastmilk freeze-drying services. With expertise in cold chain logistics, product stability, and tailored diagnostic solutions, BioAcumen is a trusted partner to research institutes, healthcare providers, and biotech companies across the region.
About Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is Singapore's lead public sector R&D agency. Through open innovation, we collaborate with our partners in both the public and private sectors to benefit the economy and society. As a Science and Technology Organisation, A*STAR bridges the gap between academia and industry. Our research creates economic growth and jobs for Singapore, and enhances lives by improving societal outcomes in healthcare, urban living, and sustainability. A*STAR plays a key role in nurturing scientific talent and leaders for the wider research community and industry. A*STAR’s R&D activities span biomedical sciences to physical sciences and engineering, with research entities primarily located in Biopolis and Fusionopolis. For ongoing news, visit www.a-star.edu.sg
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
