Downstream Processing
Pioneering Downstream Innovation for Biomanufacturing Excellence
The Downstream Processing group drives innovation in the purification and quality assessment of biotherapeutics. We develop cutting-edge purification solutions that shape the future of bioprocessing and empower both biologics research and manufacturing. Our work is strengthened by comprehensive in-process monitoring and rigorous critical quality attribute (CQA) analyses across all scales, ensuring performance, consistency, and reliability. Beyond enhancing established strategies, we actively develop next-generation purification platforms that elevate biologics production and accelerate the translation of breakthrough therapies.
Focus Areas
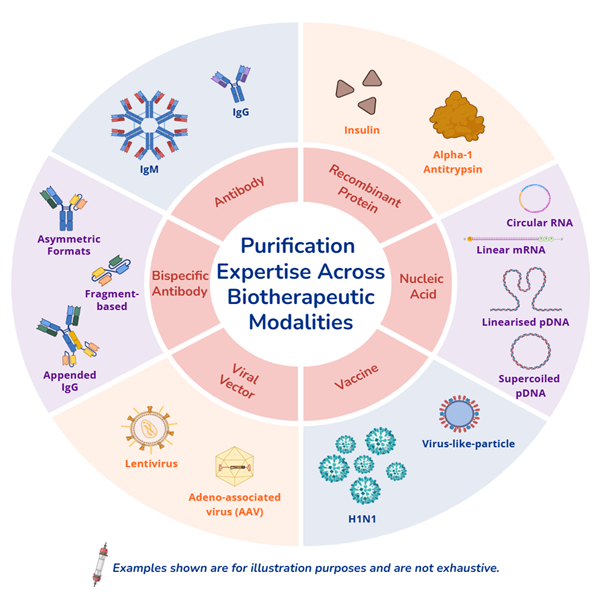
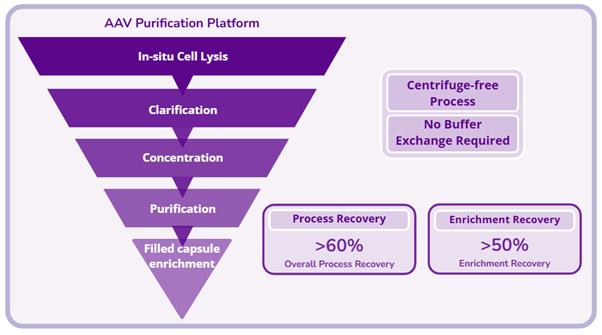
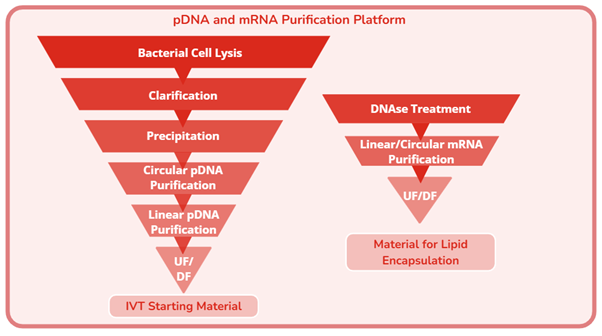
- Purification process development for protein-, cell- and nucleic acid-based (PCN) biotherapeutics, including monoclonal antibody, bispecific antibody, recombinant protein, vaccine, viral vector, and nucleic acid.
- In-process monitoring and key CQA analyses across the three main modalities of PCN biotherapeutics.
- Continuous and intensified downstream processing to enhance efficiency and scalability.
Our Capabilities
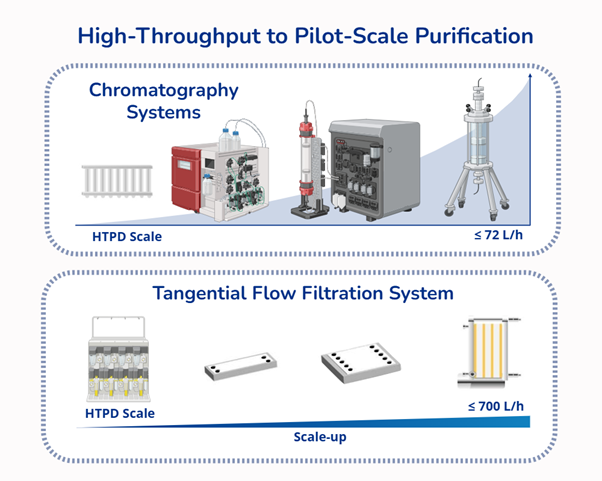
- Bridging high-throughput screening and pilot-scale purification for scalable biomanufacturing.
- Proven capabilities in purification across conventional and emerging biotherapeutic modalities.
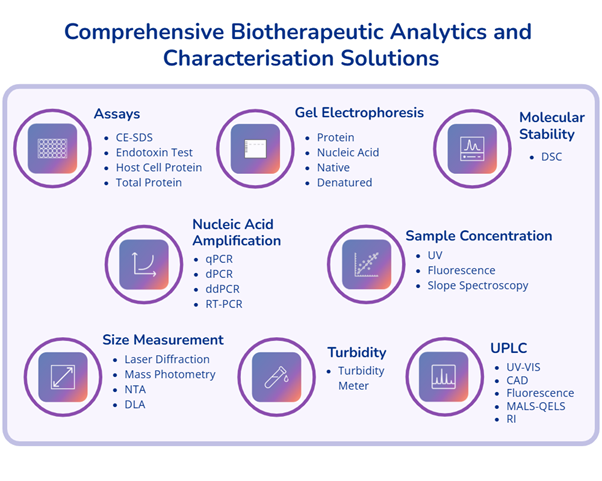
- Comprehensive analytical and characterisation solutions to enable in-depth biotherapeutic assessment across development stages.
Our Technologies
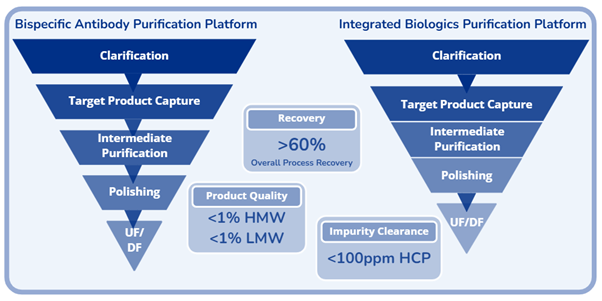
- We have developed advanced purification platforms that significantly enhance recovery, elevate product purity, and reduce manufacturing costs, enabling more efficient and scalable bioprocesses.
- Our analytical platforms provide robust in-process monitoring and rigorous final product quality control, accelerating process development while ensuring the highest standards of safety, consistency, and regulatory readiness.
- Our patents and proprietary technologies have been successfully licensed to industry partners, demonstrating strong commercial relevance and real-world impact.
The Team
.jpg?sfvrsn=d2ae7581_0)
.jpg?sfvrsn=5e6d2f8d_0)
.jpg?sfvrsn=5a61cdd5_0)
Our Track Record
Featured Publications
- Zi Ying Zheng, Wen Qin Tang, Say Kong Ng and Wei Zhang (2025) Streamlined Purification of Recombinant Alpha-1 Antitrypsin from CHO Cell Culture Using Integrated Chromatography within a Single Unit Operation. Journal of Chromatography A 1754: 466046
- Nattha Ingavat, Xinhui Wang, Yee Jiun Kok, Nuruljannah Dzulkiflie, Han Ping Loh, Eunice Leong, Kia Ngee Low, Amihan Anajao, Say Kong Ng, Yuansheng Yang, Xuezhi Bi, Wei Zhang (2025) Affinity resin selection for efficient capture of bispecific antibodies as guided by domain composition. Process Biochemistry 154: 1-11
- Zi Ying Zheng, Ashley Guan Bang Chen, Nuruljannah Dzulkiflie, Lyn Chiin Sim, Wen Qin Tang, Jiayu Leong, Wei Xuan Ler, Dawn Leong, Jake Chng, Norhaizat Bin Abdul Manan, Ian Walsh, Say Kong Ng, Maarten Pennings and Wei Zhang (2025) Quality by Design in continuous bioprocessing: Investigating the impact of critical process parameters of multi-column chromatography during steady-state and transient phases. Journal of Chromatography A 1742: 465649
- Yin Yin Siew and Wei Zhang (2025) Removing immunogenic double-stranded RNA impurities post in vitro transcription synthesis for mRNA therapeutics production: A review of chromatography strategies. Journal of Chromatography A 1740: 465576
- Xinhui Wang, Nattha Ingavat, Jia Min Liew, Nuruljannah Dzulkiflie, Han Ping Loh, Yee Jiun Kok, Xuezhi Bi, Yuansheng Yang and Wei Zhang (2024) Effects of molecule hydrophobicity and structural flexibility of appended bispecific antibody on Protein A chromatography. Journal of Chromatography A 1731: 465206.
- Serene Chen, Hoi Kong Meng, Yang Yuansheng and Zhang Wei (2022) Excellent removal of knob‑into‑hole bispecific antibody byproducts and impurities in a single‑capture chromatography. Bioresources and Bioprocessing 9: 72
- Siew Yin Yin and Zhang Wei (2021) Downstream processing of recombinant human insulin and its analogues production from E. coli inclusion bodies. Bioresources and Bioprocessing 8: 65.
- Serene W Chen, Wei Zhang (2021) Current trends and challenges in the downstream purification of bispecific antibodies. Antibody Therapeutics 4(2): 73–88.
- Duy Tien Ta, Kai Ling Chu, Nur Izzati Bte Soonaan, Christine Chin, Say Kong Ng and Wei Zhang (2021) vA new and simplified anion exchange chromatographic process for the purification of cell-grown influenza A H1N1 virus. Separation and Purification Technology 263: 118412.
- Serene W Chen, Darryl Tan, Yuan Sheng Yang and Wei Zhang (2020) Investigation of the effect of salt additives in Protein L affinity chromatography for the purification of tandem single-chain variable fragment bispecific antibodies. mAbs 12(1): 1718440
Connect with Us
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)
