Overview

-
Translation Diagnostics Laboratory
Laboratory of Translational Diagnostics at GIS promotes the smooth transition from basic research to practical innovations. We are equipped with the most advanced technologies for research and development in genomics applications.
Laboratory of Translational Diagnostics was constructed in compliance with ISO 13485 and certified by TÜV Rheinland in December 2014. It was the first laboratory in GIS to achieve certification. This positioned us as a preferred partner for strategic collaborations with local and international stakeholders.
-
Clinical Diagnostics Laboratory
POLARIS (Personalized OMIC Lattice for Advanced Research and Improving Stratification) was established by A*STAR in 2013 to pilot the application of clinical genomics in the treatment and diagnosis of medical diseases in Singapore and the region. In partnership with the hospitals and local institutions (such as SNEC, NCCS, SGH, KKH, NUHS, BTI, and EDDC), we have developed and launched tests which can provide specific actionable information to patients and doctors. By harnessing the power of genetics and technology, we can make medical genetics affordable and accessible for everyone, improving healthcare for the patient population in Singapore and the region.
POLARIS is very proud and honoured to become the first Next Generation Sequencing clinical laboratory in the South East Asia region to achieve the College of American Pathologists (CAP) accreditation. The CAP Laboratory Accreditation Program certifies the entire spectrum of laboratory test disciplines with the most rigorous requirements, and is recognised as the gold standard in Laboratory Accreditation, assuring that patients receive the highest standard of care in laboratory testing. POLARIS has also been licensed under the Private Hospitals & Medical Clinics (PHMC) Act, to meet the regulatory requirements in Singapore.
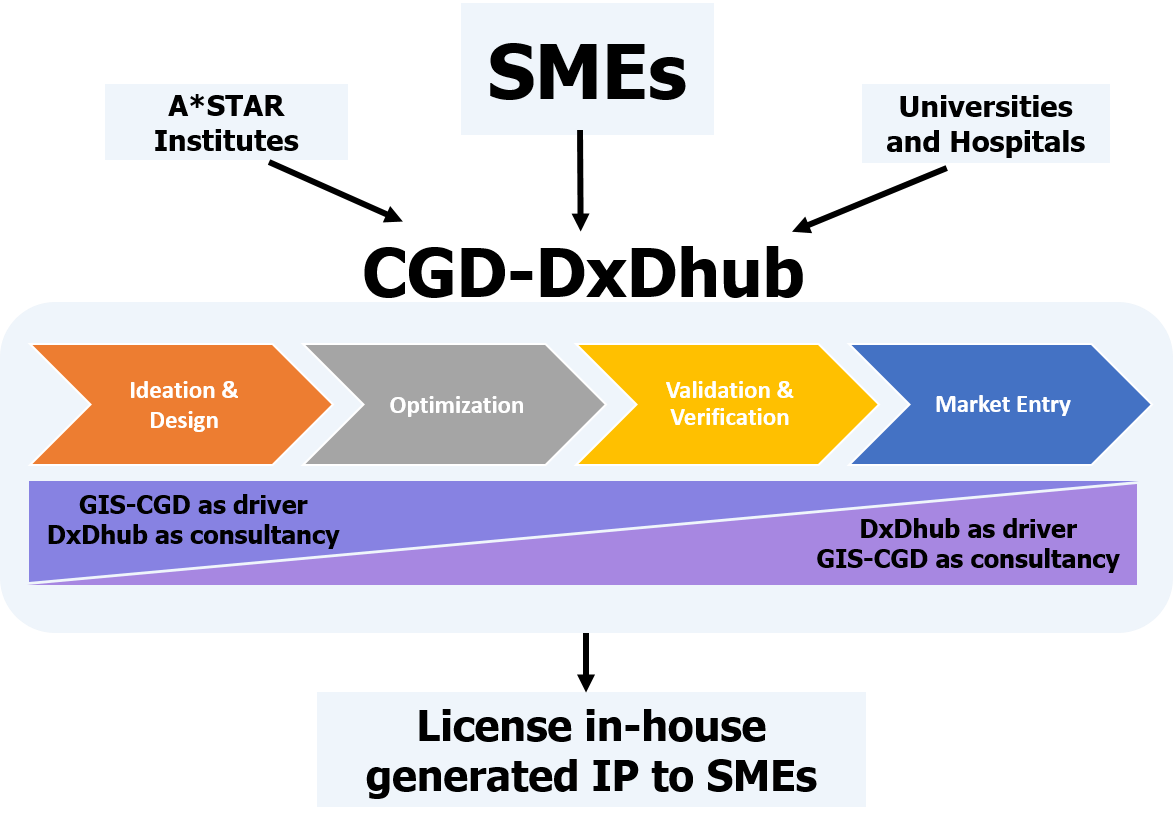
At CGD we offer an integrated suite of services from assay development to commercialization with active engagement with other A*STAR RIs, SMEs, universities and hospitals. We also offer our informatics service to better understand your data and help you achieve your goals.
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM

