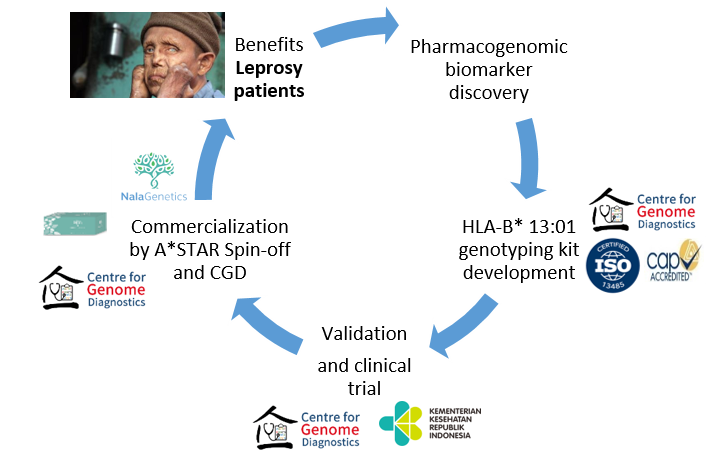
Success Stories
Clinical Diagnostic Lab
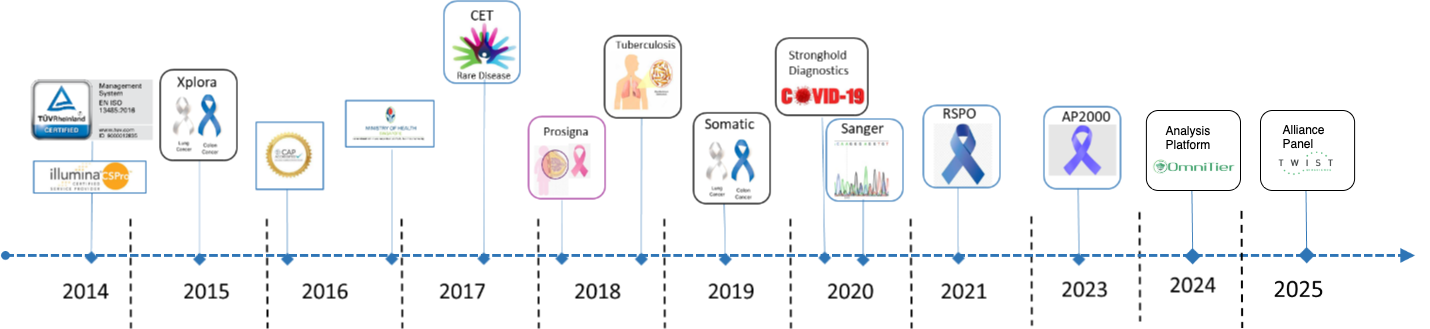
- Development and validation of multiplex RT-qPCR based RSPO Gene Fusion Test. It is a clinical trial assay (CTA) specifically designed for the qualitative detection of recurrent RSPO gene fusions .. Read More >>
Development and validation of multiplex RT-qPCR based RSPO Gene
Fusion Test. It is a clinical trial assay (CTA) specifically designed
for the qualitative detection of recurrent RSPO gene fusions in
formalin-fixed paraffin-embedded (FFPE) or fresh frozen colorectal cancer
(CRC) tissue. This was part of an early-phase clinical trial of
ETC-1922159 (Phase 1B) to support the selection of CRC patients for whom
ETC-
1922159 treatment is being considered. Presence of RSPO fusions
in CRC patients was shown to be a positive predictive biomarker for
ETC-1922159 treatment.
- As part of the contribution towards the national response to the
COVID-19 pandemic, A*STAR set up the Stronghold Diagnostics Laboratory
(SDL), .. Read More >>
Due to the urgent requirements to (a) ramp up sample volume for testing and (b) regularly update COVID-19 test results to MOH, POLARIS members in SDL instrumentally established the use of Laboratory Information Management Systems (LIMS) with integration to the National Electronic Health Record (NEHR) and COVID-19 database, enabling real-time results contribution. SDL processed 1 million samples in 2 years with results transmitted to NEHR automatically resulting in fast-turn-around time (TAT), one of the important components in fighting COVID-19 virus spread
- A germline panel for clinical exome sequencing test (CET)
was launched in 2017. This was jointly developed with KKH and NUHS to
support clinical geneticists under ... Read More >>
curation and Sanger sequencing validation. This test is now available for widespread clinical use.
- In conjunction with Parkway Medical, a Focused Cancer panel (<70 genes) (Somatic) was launched in 2019.
- Stemming from our successful pilot studies in a national project involving NUHS, CTBL (Central TB Lab, SingHealth), SSHSPH (NUS),and TTSH, DNA samples are sent to POLARIS for whole genome sequencing of tuberculosis (TB) as a service.
- POLARIS is also supporting research projects in conjunction with SGH for the systemic genotyping and phenotyping of inherited cardiac conditions.
- Breast Cancer Prognostic Gene Signature assay using NanoString Technology (Prosigna) was launched in 2018.
- A germline panel for familial adenomatous polyposis was validated and launched in 2016.
- A plasma-based EGFR Entrogen test was launched in 2016.
- Somatic solid tumour panel comprising 29 genes was launched in 2015.
- Large Cancer panel comprising over 700 genes (Xplora) was launched in 2015.
Translational Diagnostics Lab
From LAB to LIFE
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM