CGD participating in Multinational clinical trial for Made-in-Singapore cancer drug, ETC-159, by partnering with EDDC and DxD Hub


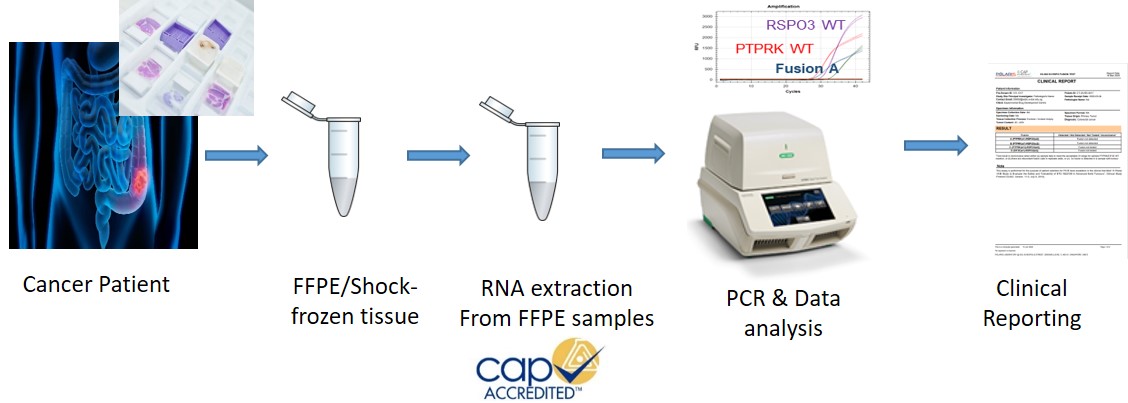
CGD together with Experimental Drug Development Centre (EDDC) and Diagnostics Development (DxD) Hub, have developed a novel diagnostic test to identify a particular subgroup of colorectal cancer patients predicted to respond well to ETC-159 due to the presence of gene fusions involving R-spondin genes (RSPO2 or RSPO3) in their tumours. POLARIS, CGD’s CAP accredited lab validated the novel diagnostics test in agreement with the US FDA.
RSPO Gene Fusion Test Kits (Fusion A, B, C and D) manufactured at CGD’s ISO 13485 certified lab are being used for the ongoing Phase 1B trial for cancer drug ETC-159.
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM

