Pioneering T-cell Immunotherapy for Liver Cancer
Lion TCR’s Breakthrough in Hepatitis B-related Cancer Treatment
The objectives
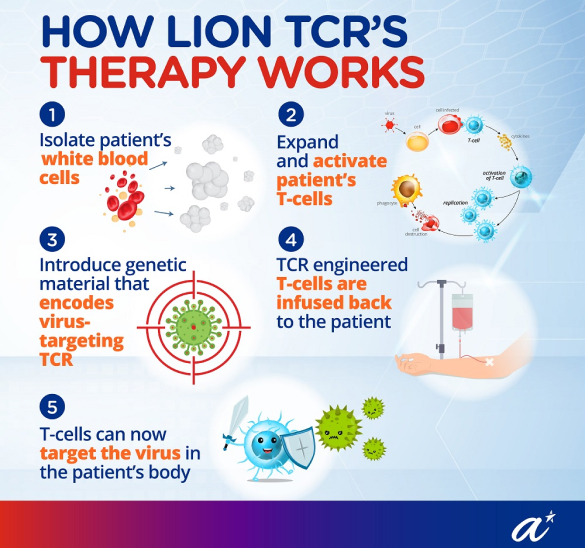
Developing Innovative T-cell Immunotherapy
Fast-Tracking Clinical Development
Expanding Intellectual Property and R&D Capabilities
The impact
Lion TCR’s innovative approach to liver cancer treatment has had a profound impact on the field of immunotherapy, particularly for patients with Hepatitis B-related liver cancer. By developing the world’s first HBV-specific TCR-T cell therapy, Lion TCR is paving the way for new treatment modalities that go beyond traditional options like surgery and liver transplantation. This breakthrough offers hope to patients with few alternatives, especially those with recurrent or late-stage disease.
Through the partnership with A*STAR and the T-Up programme, Lion TCR has accelerated its research and development efforts, bringing therapies from lab-scale research to clinical trial-ready products. The FDA Fast Track Designation for their lead therapy, LioCyx-M004, underscores the significance of their work and highlights the potential to meet urgent medical needs. This designation not only expedites the approval process but also supports Lion TCR’s mission to provide effective cancer treatments in a timely manner.
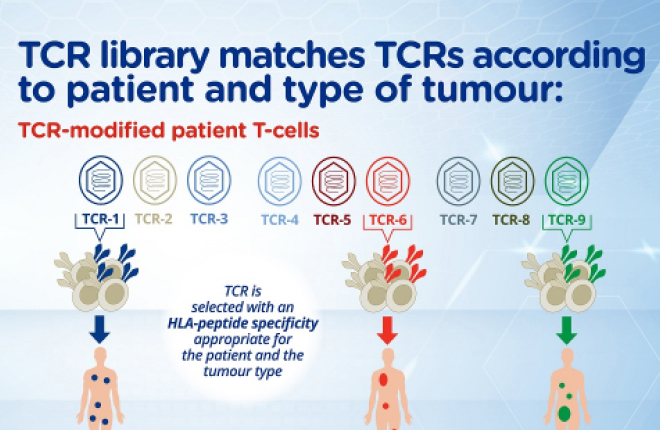
Moreover, the expertise gained from A*STAR has enabled Lion TCR to build the largest patented HBV-specific TCR library, covering approximately 80 percent of the global population. This advancement positions Lion TCR as a global leader in liver cancer immunotherapy, capable of addressing the needs of a wide patient base. By leveraging technology licensing and strategic partnerships, Lion TCR has created a robust platform for further innovation and expansion.
Lion TCR’s work represents a vital step forward in cancer treatment and serves as a model for how collaboration and cutting-edge research can lead to life-changing therapies. As they continue to grow, Lion TCR remains committed to pushing the boundaries of what is possible in immunotherapy, offering new hope for patients around the world.
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
