
Olaf RÖTZSCHKE
Olaf_Rotzschke@a-star.edu.sgBiography
Olaf Rötzschke obtained his PhD in 1993 at the University of Tübingen, Germany, where he studied biochemistry. He started his career at the Max-Planck Institute of Biology in the group of Hans-Georg Rammensee, where he isolated the first natural T cell antigen and defined the binding motifs of MHC molecules. In 1993 he joined the group of Jack Strominger at Harvard University, carrying out studies on the immune-modulation and antigen-presentation. In 2000 he moved to the Max-Delbrück-Center in Berlin focusing on regulatory T cells (Treg) and MHC ligand-exchange. He joined SIgN as Principal Investigator in July 2008 and in 2018 became also Head of two SIgN Immunomonitoring Platforms (‘Mass Cytometry’ and ‘Multiplex Analysis of Proteins’).
Adjunct Positions
- Adjunct Associate Professor, NUS, Singapore
- Adjunct Professor, NTU, Singapore
Research Focus
Allergy and Immune-regulation
One central aspect of the current research is the functional and genetic characterization of allergy-related pathways in humans. Basis for these studies is a cohort of 600 Singaporean residents for which the group established a database comprising more than 3000 data entries per individual (Figure 1A). One of the key-results of this investigation was the identification of house dust mites (HDM) as a major public health problem in Singapore (Figure 1B). Nearly 80% of the local population were sensitized by this allergen, of which about half developed symptoms of airway-allergy symptoms of allergic rhinitis and/or asthma (Figure 1C). The dominance of dust mite allergens is evident in exceedingly strong IgE titer (Figure 1D) and the penetration of the tropical-urban environment by this allergen is so strong, that non-allergic migrants typically develop symptoms within 8 years after coming to Singapore.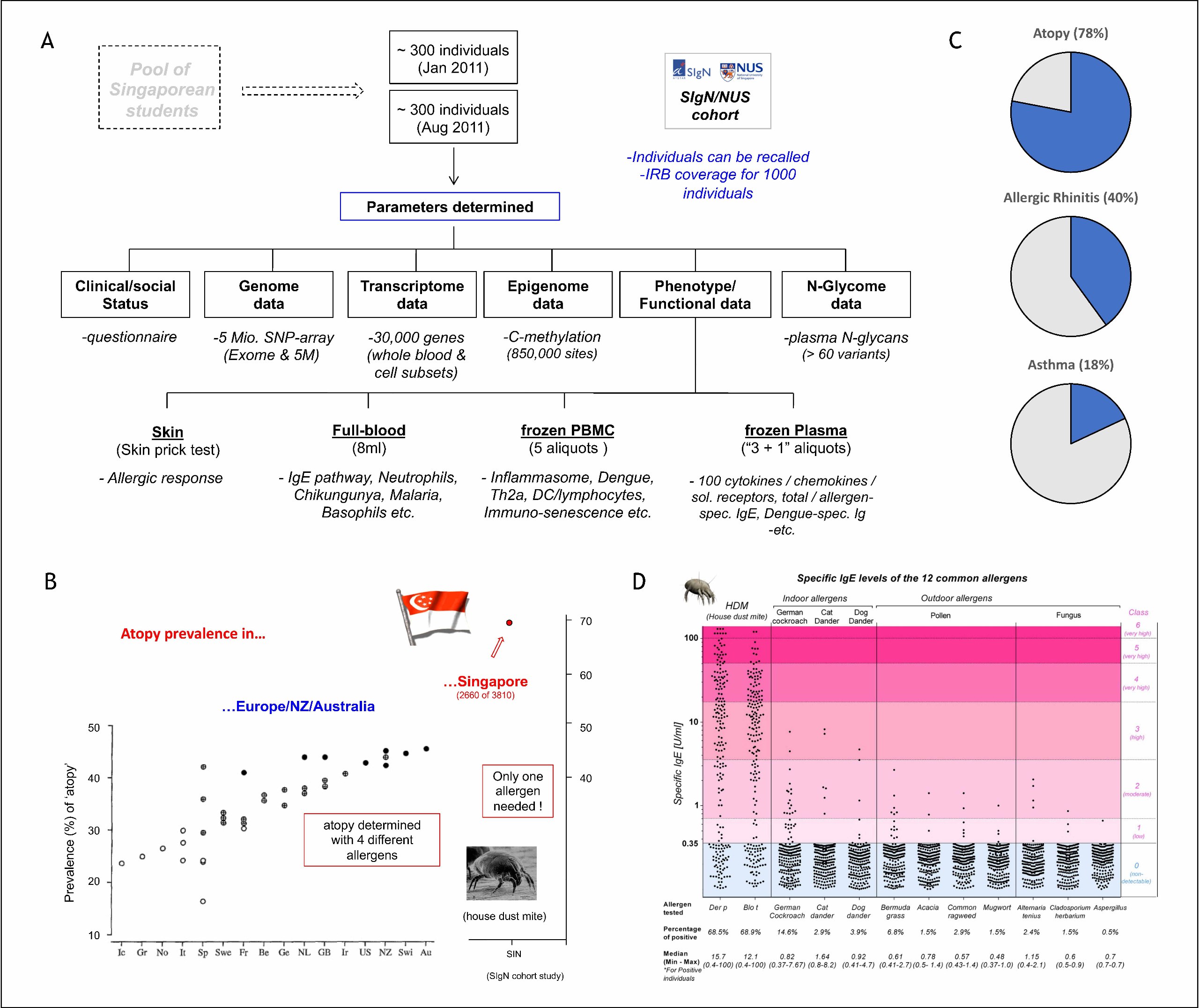
Figure 1: House dust mite-induced allergies in Singapore (Andiappan et al. 2014, Allergy 69:501; https://doi.org/10.1111/all.12364)
The central role of Basophils
Basophils are often considered to be a cell subset of minor significance. Our studies indicated however that they are instrumental in triggering an effective IgE-mediated allergen response. IgE is the only immunoglobulin that can be generated outside of germinal centres directly in the mucosal tissue. Upon stimulation with allergen/IgE-complex, basophils can support the local IgE production by providing crucial IL-4 (Figure 1A). Analysis of our cohort data revealed now that basophils can acquire an ‘anergic’ state that renders them insensitive for this stimulation (Figure 2B). This ‘basophil anergy’ is mediated by the down-regulation of SYK, a key-kinase acting as gate-keeper of the IgE/FcER1 signalling pathway. Notably, basophil anergy was found to be a mandatory transitional state separating non-responsiveness (caused by the lack of specific IgE) from the normal allergen-responsiveness of an individual. It thus acts as an important activation barrier preventing the unwanted triggering of the allergen-response pathway.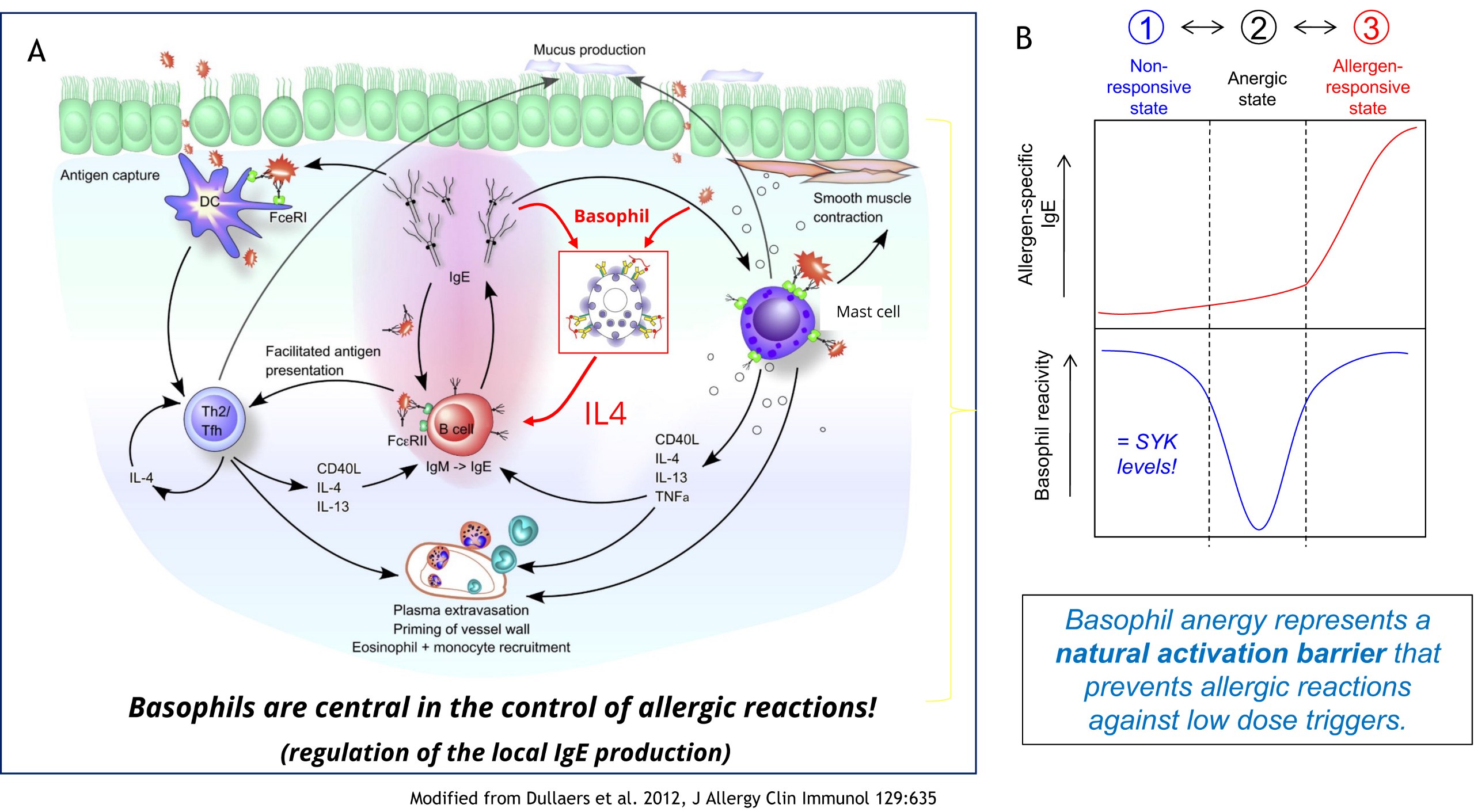
Figure 2: Basophil anergy as control element for IgE production (Puan et al. 2017, Allergy 72:373; https://doi.org/10.1111/all.12952).
Additional evidence for the crucial role basophils was derived from a study reporting a previously unknown homing defect of the cells. Flow cytometry analysis revealed that about 10% of the individuals lack the expression of CD15s on their basophils (Figure 3B). This phenotype indicates that sialyl-Lewis X (sLeX) is missing on the surface, a small glucan crucial for the evasion from the blood vessels (Figure 3A). CD15sneg basophils are found to be unable to ‘roll’ on the endothelial lining, a failure that could be traced back to genetic defects in the fucosyltransferase 6 (FUT6), which completes the sLeX synthesis by mediating the transfer of fucose residue . Using this genetic link to analyse about 500,000 annotated genomes provided by the Welcome Sanger Institute and 23andMe, respectively, we could confirm that these defects are indeed associated with a retainment of basophils within the blood vessels and a reduced itch sensitivity to mosquito bites (Figure 3C).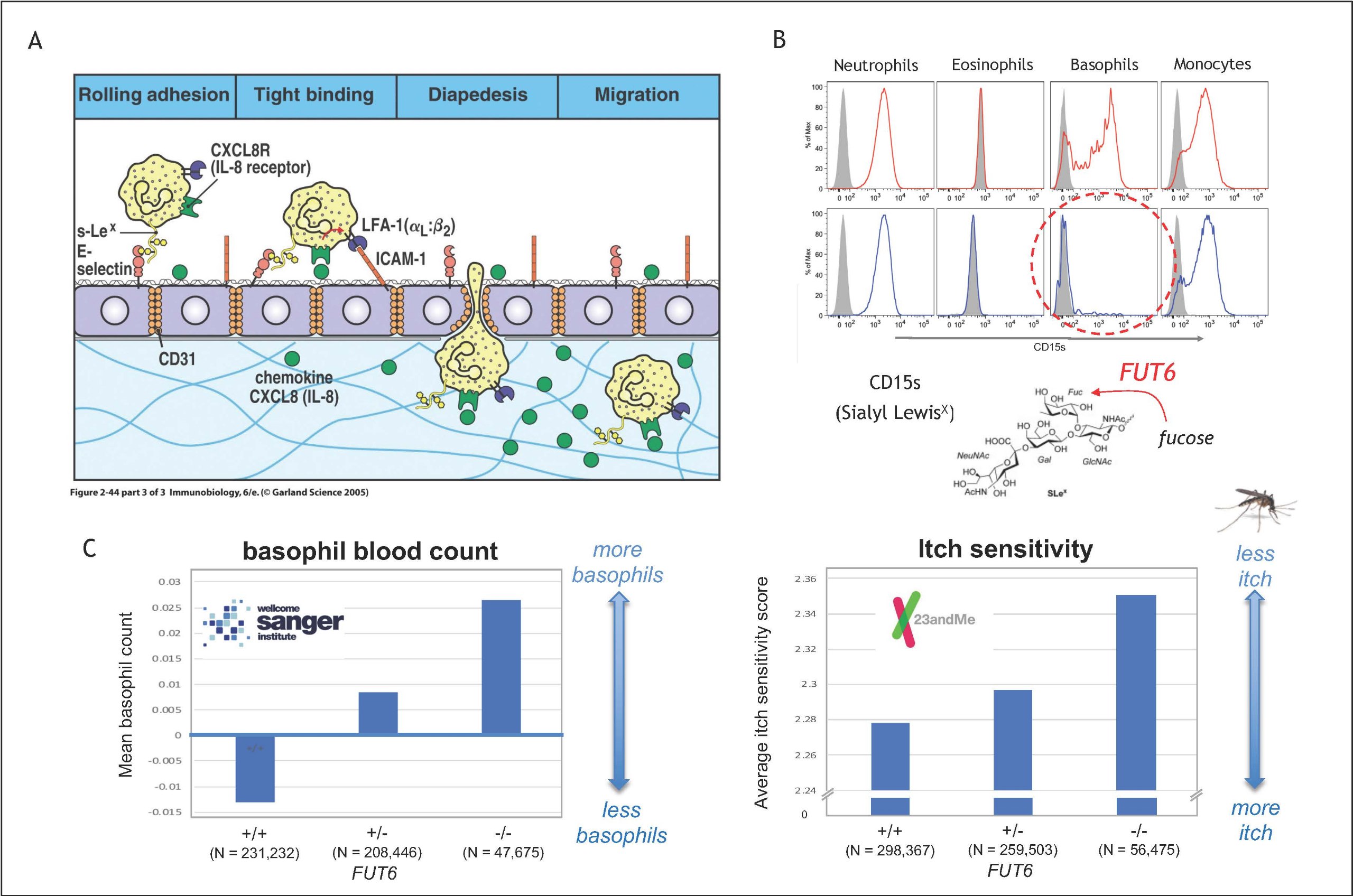
Figure 3: Effect of FUT6 deficiency on allergic reactions (Puan et al. 2021, Commun Biol. 4:832; DOI: 10.1038/s42003-021-02295-8)
T cell-based tumour immunology
Another research area more recently explored by the group is T cell-based tumour immunology. The change in focus was motivated in part by the recent success in checkpoint inhibition trials as well as by the availability of a world-class mass cytometry platform at SIgN, which also offers complex custom-made pMHC-tetramer arrays for the antigens-specific T cell tracking. In the context of the SYMPHONY OF-LCG network project, these tools are currently used for the detection and analysis of virus-specific T cells in NK/T lymphoma (NKTL), a malignancy strongly associated with ass Epstein-Bar Virus (EBV) (Figure 4).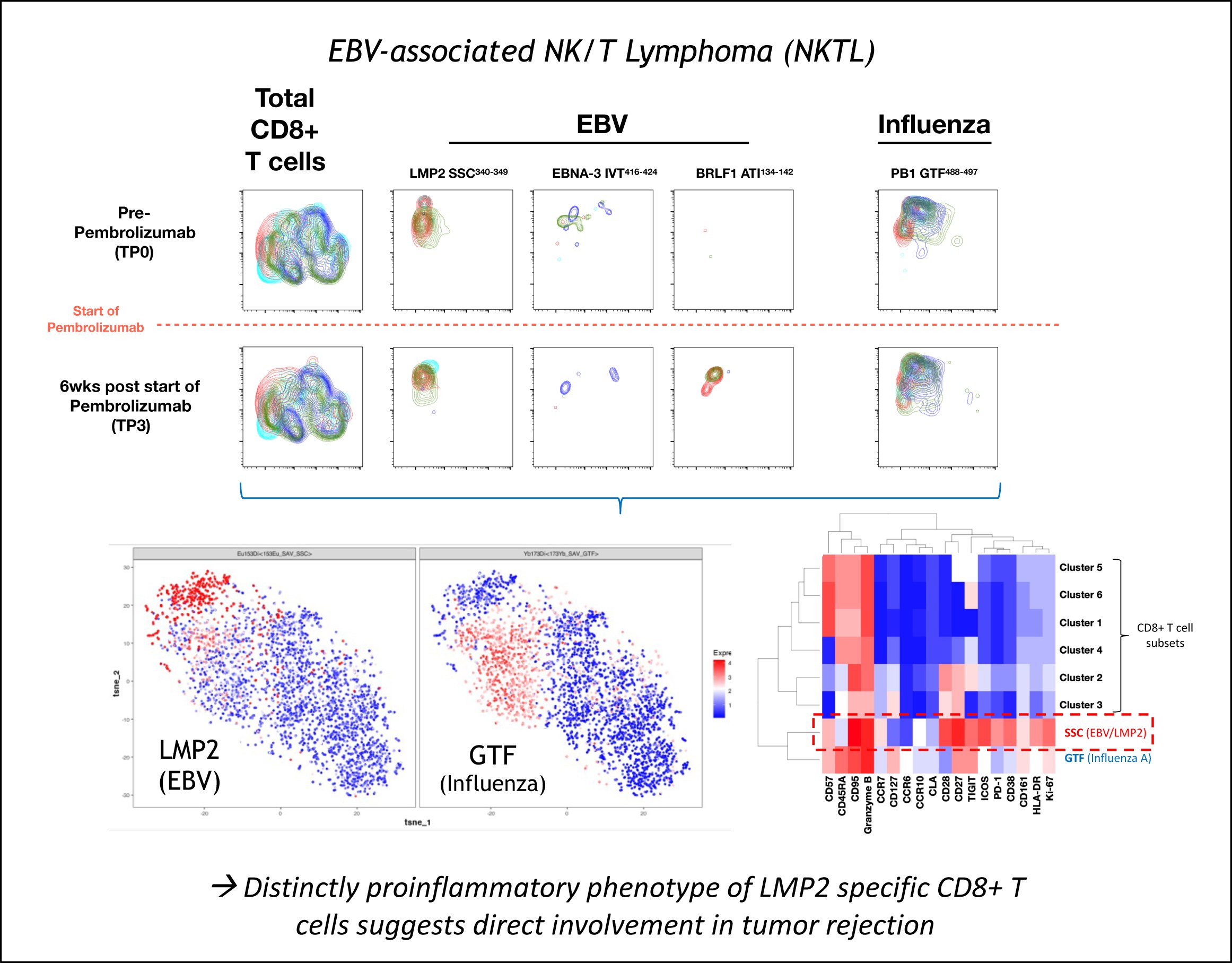
Figure 4: Phenotype of EBV- and Influenza virus-specific T cells before and after checkpoint inhibition by anti-PD1 (pembrolizumab).
While these studies strongly suggest a direct involvement of tumour-specific T cells in tumour rejection, a working pipeline to carry out therapeutic T cell vaccinations in Singapore was still missing. We therefore started to establish a national platform with the specific aim to generate and test personalized T cell vaccines. The ‘T-cell Monitoring and Vaccination Platform (T-MoVac)’ launched in March 2023 was funded as an A*STAR Industry Alignment Fund pre-positioning project (IAF-PP; H22J1a0043).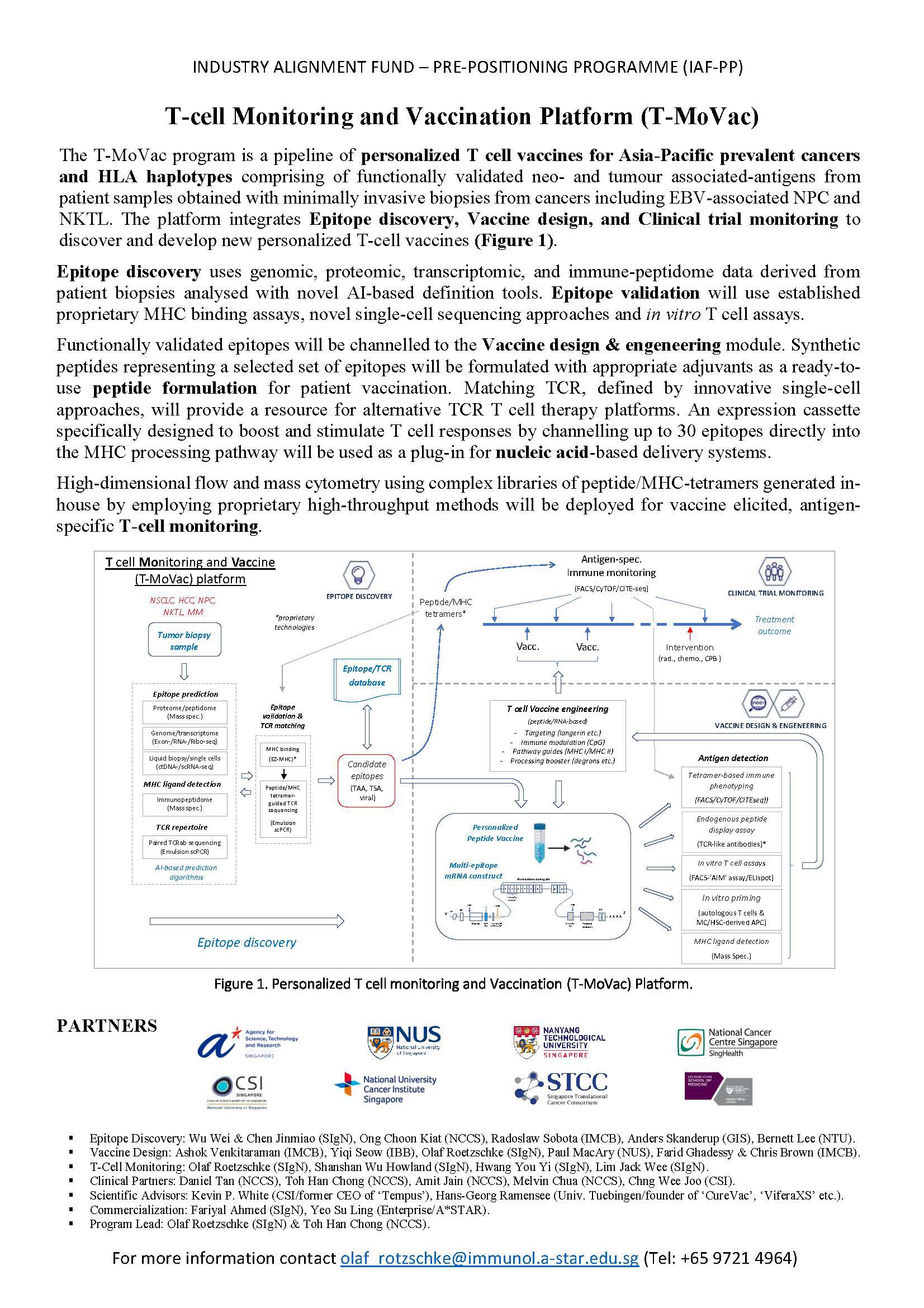
Lab Members
| Postdocs (Ph.D) | Research Officers | PhD Students |
|---|---|---|
| Wendy LEE | Ser Mei KOH | Nai Yao LAM |
| Boris San LUIS | P Lokamitra | |
Publications
Publications_Olaf Rotzschke (last updated 19 April 2024)
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
