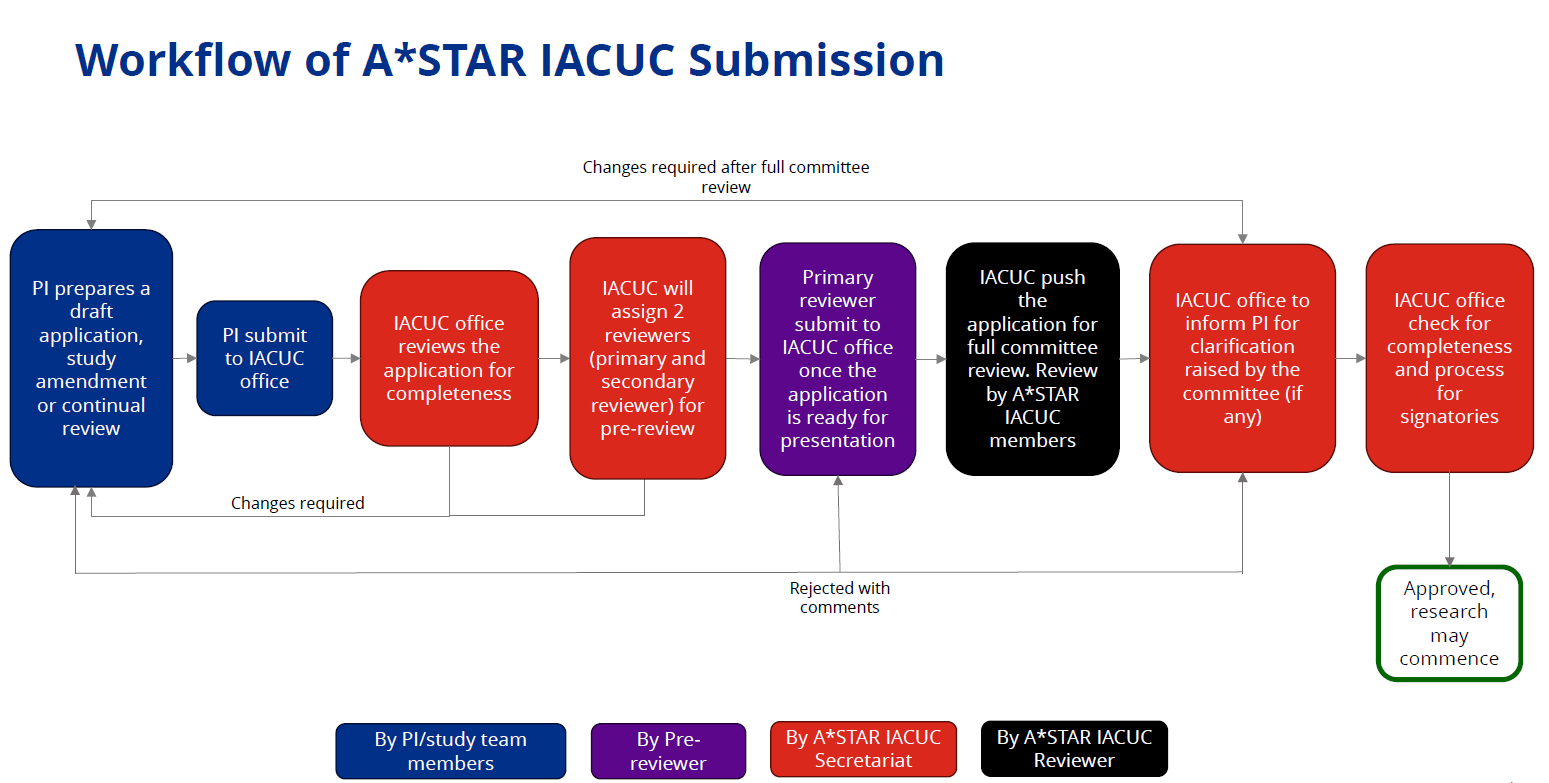
PROTOCOL SUBMISSION REVIEW
IACUC Protocol Submission
All IACUC Protocol applications, amendments and annual reviews are to be created and submitted via HARMONY (Human & Animal Research Management & Oversight system).
Both internal and external users are required to have an account created for access to the portal. PI to fill-in the “Account Creation” excel file and submit this with a copy of the staffs RCULA certificates to IACUC Office.
IMPORTANT Reminder: Accounts will be locked after 90 days of inactivity. If your account is locked, please contact IACUC Office to re-activate your account.
Refer to the user guide available below for the important pre-requisites of the portal and guide on navigating the portal.
PI to note that incomplete submission is not accepted and will be returned to the PI by IACUC Office. PI must provide the respective supporting documents during submission.
- GMAC Approval: for use of genetically modified/engineered animals
- IRB Exemption/Approval Letter: for assistance on the use of human tissues or studies involving human subjects (including use of the commercially-available human cell lines)– you may contact A*STAR HBR Office for assistance
- Risk Assessment (RA): legal requirement for all works carried out at A*STAR animal facilities – you many contact the IBC of your Institute for assistance
- Biosafety
- Mycoplasma Test: for use of human & non-rodent cells/tissues
- MAP/RAP Test: for use of mouse/rat cells or tissues. (contact BRC Vets for more information)
- Other supporting documents as per requested, such as SDS, References, Annexes etc.
Note to New Applicants: It is advisable for the first-time IACUC users to arrange a face-to-face meeting with IACUC members and BRC Veterinarians for better understanding of the process. You may do so by contacting IACUC Office.
Note to Industrial Applicants: Applications from A*STAR's industrial partners and collaborators will be reviewed under the industrial track, which aims to get the protocols being reviewed and approved within 4 weeks upon successful submission. New users may also engage in an IACUC Consultancy Service provided by InVivos Pte Ltd. Please contact enquiries@invivos.com.sg for more information.


A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
