Porous biomaterial with encapsulated microbes functions like a 'biocatalytic converter'

Science
Many cells produce enzymes that can perform complex chemical reactions efficiently. Recent advances in synthetic biology has further provided engineered strains that allow non-native producers, such as hardier microbes, to serve as hosts for a wide variety of enzymes. However, cells are dynamic systems, and typically have a limited working life as whole-cell biocatalyst, undergoing proliferation, enzyme production, and ultimately dying off in the reactor. In this work, we demonstrate that microbes encapsulated in a macroporous biomaterial maintains their biocatalytic activity, while maintaining a stable cell population for almost 2 months. This work paves the way for stable biocatalytic units that require only periodic replacement.
Societal Impact
Biocatalysis provides a greener and more sustainable alternative for synthesizing high value chemicals – including active pharmaceutical ingredients (API) – when compared with conventional chemical synthesis. Furthermore, using whole cells instead of purified enzymes as biocatalysts can also avoid the costs associated with the purification process. By restricting cell growth without affecting their enzymatic activity, our method allows us to create catalytic units that can incorporate multiple strains with different enzymatic activities in a single unit, thereby enabling complex multi-step reaction cascades to be performed. This will greatly expand the possibilities for whole-cell biocatalysis.
Technical Summary
Microbial cells engineered to express one or more enzymes can serve as whole-cell biocatalysts. In many instances, molecules that would otherwise be synthesized using conventional chemical synthesis have relatively low molecular weights. This allows them to diffuse into cells, where they can be enzymatically modified, and back out into the surrounding media. However, the cells in culture are highly dynamic, and transition from proliferative phase (where their numbers increase) to a production phase (where more resources are directed towards protein expression), and finally die off. This makes control of their catalytic activity challenging.
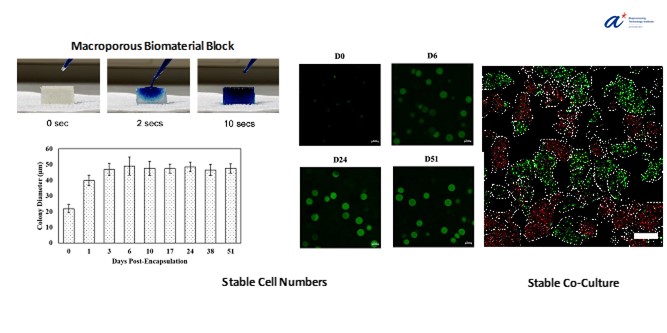
By encapsulating the cells in hydrogel microparticles before crosslinking them into a macroporous block, we have been able to stop the cells from growing appreciably after a few days, thus maintaining a stable population. The hydrogel microparticles are also small enough to permit ample access to the nutrients in the media, as well as allow transport of enzymatic substrates through the hydrogel matrix, into the cells, where they were successfully modified. These microbe-laden hydrogel blocks were successfully maintained in culture for almost 2 months. Furthermore, we demonstrate that by forming our porous blocks from particles containing different microbes, stable co-culture of different strains can be achieved. This paves the way for combining multiple steps of a reaction cascade into a single biocatalytic unit, thus simplifying the bioprocessing workflow.
References
Zhang, Jingyi, et al. "Hyper-porous encapsulation of microbes for whole cell biocatalysis and biomanufacturing." Microbial Cell Factories 24.1 (2025): 48. https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-025-02675-3
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)