Our Capabilities
Extensive and in-depth expertise in purification of traditional and emerging modalities

Bispecific Antibody
- Fragment based
- Asymmetric formats
- Appended IgGs
Antibody
- IgG
- IgM

Nucleic Acid
- Linear mRNA
- Circular mRNA
- Supercoiled pDNA
- Linearised pDNA

Recombinant Protein
- Insulin
- Alpha-1 Antitrypsin
Vaccine
- H1N1
- Virus-like-particle

Viral Vector, Gene Therapy
- AAV 1, 2, 5, 6, 8, 9 and DJ
Downstream process development
.jpg?sfvrsn=965a96ee_0)
- Purification process development from high throughput scale to pilot scale for bio-products
Analytics and Characterisation

UHPLC
- UV-Vis
- CAD
- Fluorescence
- MALS
- RI
Sample concentration
- UV
- Fluorescence
- Slope Spectroscopy

Nucleic acid amplification
- qPCR
- dPCR
- ddPCR
- RT-PCR

Assays
- CE-SDS
- Endotoxin test
- Host cell protein
- Total protein

Size measurement
- NTA
- DLS

Turbidimetry
- Turbidity meter

Molecular stability
- DSC
Gel electrophoresis
- Protein/ nucleic acid
- Native/denatured
Our Technologies
- We have established purification platforms with improved recovery, product purity and increased cost-effectiveness for both traditional and emerging modalities
- We offer comprehensive analytical platforms for in-process monitoring and final product QC to accelerate process development and ensure product quality and safety.
- Several patents and technological know-hows have been licensed to companies
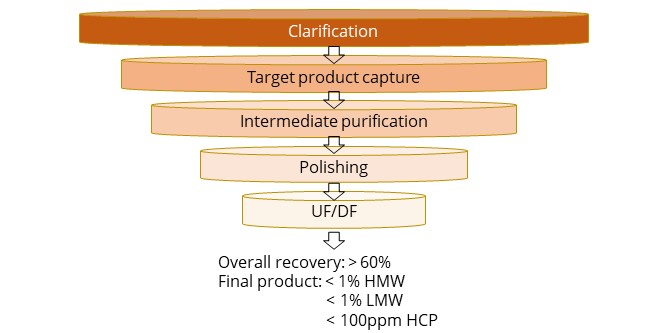
Bispecific antibody purification platform
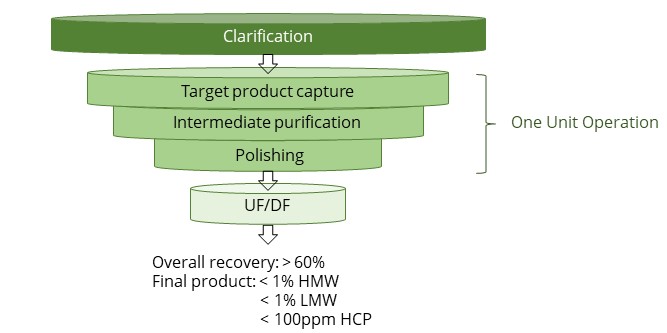
Integrated biologics purification platform
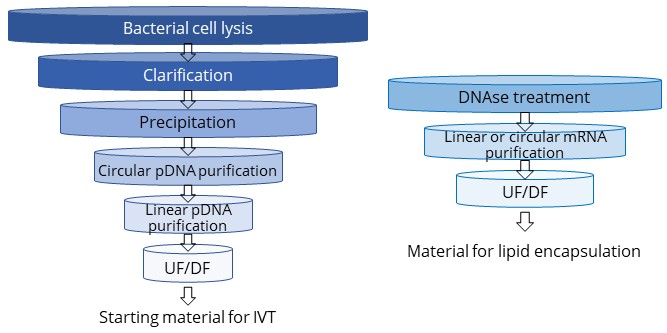
pDNA and mRNA purification platform
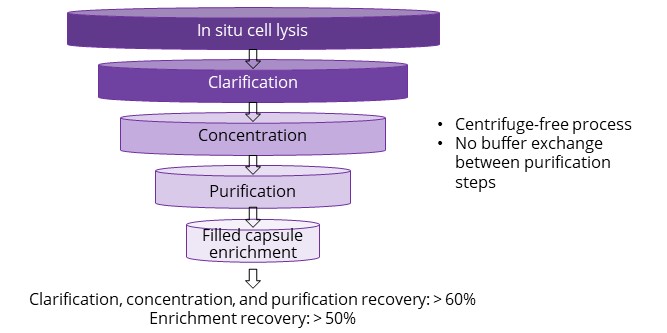
AAV purification platform
Our Track Record
Featured publications:
- Serene W Chen, Zi Ying Zheng, Farouq Bin Mahfut, Yuansheng Yang, Masahiro Ogino, Kazuo Okada, Kohei Sato and Wei Zhang (2023) Leveraging an advanced simulated moving bed approach to achieve 3-component separation for enhanced impurity removal in a non-affinity cation exchange capture step. PLoS ONE 18(1): e0280760
- Serene Chen, Hoi Kong Meng, Yang Yuansheng and Zhang Wei (2022) Excellent removal of knob‑into‑hole bispecific antibody byproducts and impurities in a single‑capture chromatography. Bioresources and Bioprocessing 9: 72
- Siew Yin Yin, Amrita Rai, Han Bin Pek, Dave Siak-Wei Ow and Zhang Wei (2021) New and efficient purification process for recombinant human insulin produced in Escherichia coli. Applied Microbiology and Biotechnology 105(24): 9137-9151
- Duy Tien Ta, Kai Ling Chu, Nur Izzati Bte Soonaan, Christine Chin, Say Kong Ng and Wei Zhang (2021) A new and simplified anion exchange chromatographic process for the purification of cell-grown influenza A H1N1 virus. Separation and Purification Technology 263: 118412
- Serene W Chen, Darryl Tan, Yuan Sheng Yang and Wei Zhang (2020) Investigation of the effect of salt additives in Protein L affinity chromatography for the purification of tandem single-chain variable fragment bispecific antibodies. mAbs 12 (1): 1718440
The Team

Dr Zhang Wei
zhang_wei@bti.a-star.edu.sg
Principal Scientist I
PhD in Chemical and Pharmaceutical Engineering (2011), Singapore-MIT Alliance, National University of Singapore, Singapore
Research Focus / Interest- Purification process and analytics development for biologics, vaccines, cell and gene therapy, and nucleic acid therapy
- Continuous and intensified downstream processing
- Process scaling up and technical transfer to GMP facility

Dr Siew Yin Yin
siew_yin_yin@bti.a-star.edu.sg
Senior Scientist II
PhD in Pharmacy (2016), National University of Singapore, Singapore
Research Focus / Interest- Biotherapeutics purification and characterization

Dr Nattha Ingavat
nattha_ingavat@bti.a-star.edu.sg
Senior Scientist I
PhD in Department of Chemistry (2017), Johns Hopkins University, Maryland, USA
Research Focus / Interest- Antibody (Bispecific antibodies) purification and characterization

Dr Patric Chua
patric_chua@bti.a-star.edu.sg
Scientist
PhD in Microbiology (2017), Monash University, Australia
Research Focus / Interest- Recombinant Adeno-associated Virus (AAV) purification and characterisation
.png?sfvrsn=1a7df424_3)

