A robust and sensitive analytical assay to quantify multiple free ADC payloads

Science
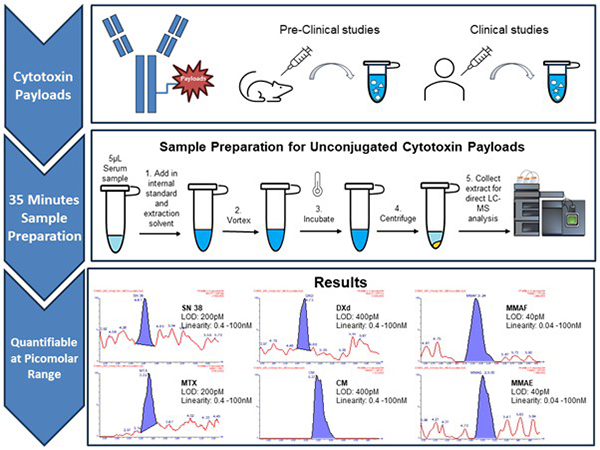
An Antibody-Drug Conjugate (ADC) is a targeted cancer treatment that combines a monoclonal antibody with a chemotherapy drug (known as the “drug payload”). It ideally delivers the highly toxic drug payload directly to cancer cells, preventing healthy cells from being damaged. Therefore, it is critical to assess the stability of the ADC to minimise premature drug release after being administered in pre-clinical and clinical studies. This highlights the need for a sensitive and robust analytical assay to accurately quantify multiple ADC free drug payloads simultaneously, supporting the safety and efficacy evaluation of ADC candidates.
Societal Impact
Cancer is one of the top causes of death worldwide. According to the 2021 World Health Organization report, there is an estimation of 19.3 million new cancer cases occurring in 20201. This is a driving force for the rapid development of ADCs as a promising class of biotherapeutics for targeted cancer therapy, enabling the selective treatment of cancer cells. To date, fifteen ADCs have been approved by U.S. Food and Drug Administration (FDA), with many more entering clinical trials2,3. Accurately quantifying the premature release of free drug payload from ADCs aids in optimising the design of ADC structure to minimise potential side effects, ensuring that patients receive effective therapeutic doses and potentially improving treatment outcomes.
Technical Summary
A highly sensitive and robust analytical assay, based on liquid chromatography-mass spectrometry (LC-MS) was developed to characterise and quantify 6 different unconjugated ADC payloads (SN-38, MTX, DXd, MMAE, MMAF, Calicheamicin) in serum samples. This assay has been developed and validated in accordance with ICH guidelines. The demonstrated assay workflow is amenable to automation for high-throughput analysis of free ADC payloads in in-vitro and in-vivo studies. This assay was also successfully applied to a pharmacokinetic study to determine free MMAE levels in mice after ADC administration. Lastly, we hope that the development of this sensitive LC-MS assay for simultaneous quantification of 6 ADC payloads, would facilitate and support future development of multi-payload ADCs.
References
1. Mak, S.Y., Chen, S., Fong, W.J. et al. A simple and highly sensitive LC–MS workflow for characterization and quantification of ADC cleavable payloads. Sci Rep 14, 11018 (2024). https://doi.org/10.1038/s41598-024-61522-4.
2. Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
3. Nguyen, T. D., Bordeau, B. M. & Balthasar, J. P. Mechanisms of ADC toxicity and strategies to increase ADC tolerability. Cancers (Basel) 15, 713 (2023).
4. Wang, Z., Li, H., Gou, L., Li, W. & Wang, Y. Antibody–drug conjugates: Recent advances in payloads. Acta Pharm. Sin. B. https://doi. org/ 10. 1016/j.apsb.2023. 06. 015 (2023).
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)