Anticancer nanocomplexes: mitigation of drug-resistance with increased tumor targeting and better biocompatibility
Science
The development of drug resistance is one of the most common reasons for the poor recovery of cancer patients. Recently, a new class of macromolecular drugs such as positively charged biopolymers and peptides has been proposed to overcome this problem. However, positively charged biopolymers are also toxic to healthy cells and tissues. To this end, we developed negatively charged biopolymers to form nanoparticles with a positively charged anticancer biopolymer called nanocomplexes. In this paper, we reported that these nanocomplexes stayed in the body longer, were more biocompatible and could target tumors better than the anticancer biopolymer alone 1.
Societal Impact
Nanotechnology has the potential to overcome limitations of conventional small molecule drugs by changing the way of how drugs are delivered to the target, such as reducing drug side effects by preventing it from acting on healthy cells. To date, there are at least 8 approved nanoparticle formulations for cancer treatments with many more in clinical trials. However, nanoparticle formulations that carry conventional drugs often do not address the problem of drug resistance because the formulations do not alter the drug’s mechanism of action. Cancer cells can still develop resistance to these drugs when they are released from the nanoparticles. Therefore, we need to take a different approach to develop new treatments for cancer. Previous studies have shown that cancer cells were unable to develop drug resistance to anticancer biopolymers. However, like conventional drugs, they could be toxic to healthy cells. In this study, we developed nanoparticle formulations containing an anticancer biopolymer and showed that in mice the nanoparticles were effective in suppressing tumor growth by 32-56% and reduced injection site toxicity without causing any observable damage to the major organs such as liver and kidneys.
Technical Summary
We have previously reported guanidinium-based polycarbonates as anticancer agents 2. In this study, the anticancer biopolymer is a polycarbonate with 20 repeating units of a guanidinium monomer containing a butyl group as a hydrophobic spacer. New anionic block copolymers, comprising of polyethylene glycol and benzoic acid-functionalized polycarbonate were synthesized using organocatalytic ring-opening polymerization. In addition, to increase the targeting ability of the nanoparticles, biotin was conjugated on one end of the anionic polycarbonate. The uniformly dispersed nanocomplexes were formed by self-assembly in aqueous solution, with hydrodynamic diameters of less than 130 nm and anticancer biopolymer loading levels of 38-49%.
The nanocomplexes were effective at inhibiting the growth of MCF-7/ADR, a doxorubicin-resistant human breast cancer cell line, with a low half-maximal inhibitory concentration of 24 µg/mL. The nanocomplexes entered the cancer cells via endocytosis and killed the cells by triggering apoptosis. In mice, the nanocomplexes increased the plasma half-life of the anticancer polycarbonate from 1 hour to 6-8 hours, indicating that the nanocomplex formulation prolonged the plasma circulation of the anticancer polycarbonate for better accumulation in tumor tissue. Mice that were administered with the anticancer polycarbonate on its own experienced weight loss and injection-site toxicity. In contrast, mice treated with the nanocomplexes had an average of 43% tumor suppression and no pathological abnormalities as observed from H&E-stained sections of the kidneys and liver.
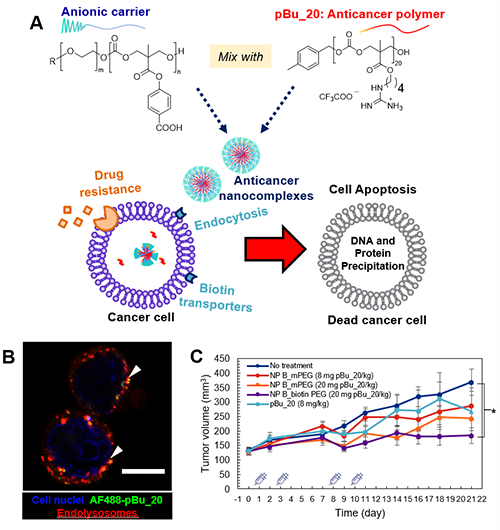
Figure 1. (A) Scheme of the nanocomplexes targeting drug-resistance cancer cells that overexpress biotin transporters. After the nanocomplexes were internalized, the anticancer polymers were free to bind to DNA and proteins in the cell which triggered cell death by apoptosis. (B) Intracellular distribution of NP B_biotinPEG (nanocomplexes) in BT-474 human breast cancer cells. (C) Tumor volume in mice over time after various treatments. For more details, please refer to the publication1. Copyright Wiley-VCH GmbH. Reproduced with permission.
References
- [1] Leong, J., Tay, J., Yang, S., Yang, C., Tan, E. W. P., Wang, Y., Tan, B. Q., Hor, S., Chua, Y. H., Tan, J. P. K., Chen, Q., Hedrick, J. L., Yang, Y. Y., Nanocomplexes of Biodegradable Anticancer Macromolecules: Prolonged Plasma Half-Life, Reduced Toxicity, and Increased Tumor Targeting. Adv. Healthcare Mater. 2023, 2201560. https://doi.org/10.1002/adhm.202201560
- [2] Tay, J., Zhao Y., Hedrick J. L., Yang Y. Y., Theranostics 2021,11, 8977.
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)