In-depth insights into continuous biomanufacturing downstream processing

Science
Continuous biomanufacturing (CM) represents a major advancement in biomanufacturing, offering increased productivity and cost reduction. Multi-column chromatography (MCC) is a key unit operation in CM, playing a critical role in downstream processing. In this study, we employed a full factorial design to examine the impact of critical process parameters during both steady-state and transient phases of MCC. Our findings provide deeper insights into the process dynamics of CM.
Societal Impact
Compared to conventional batch-mode biomanufacturing, CM offers several advantages, such as enhanced efficiency, a smaller footprint, optimized resource utilization, and greater flexibility and scalability. However, a key technical challenge lies in the dynamic and adaptive management of these complex processes, particularly in downstream processing, which involves multiple unit operations. This study provides in-depth insights into continuous downstream processing, potentially facilitating the broader adoption of CM.
Technical Summary
Given the complexities of continuous bioprocessing, it is essential to thoroughly evaluate the unique process parameters of MCC and their potential impacts. In this study, we investigated the combined effects of (i) loading densities and (ii) residence time (RT) during both steady-state and transient phases (start-up, shut-down, and perturbation) on key process attributes and critical quality attributes. Our findings showed that MCC operation under static control resulted in a maximum UV variation of 30% between the start-up phase and steady state. Additionally, eluate concentration exhibited a moderate increase (25% deviation) when transitioning from start-up to steady state but dropped significantly (93% deviation) when moving from steady state to the shut-down phase. However, variations in product quality could potentially be mitigated through product diversion or pooling with high-purity eluates from steady-state operation, demonstrating the robustness of MCC in maintaining consistent product quality.
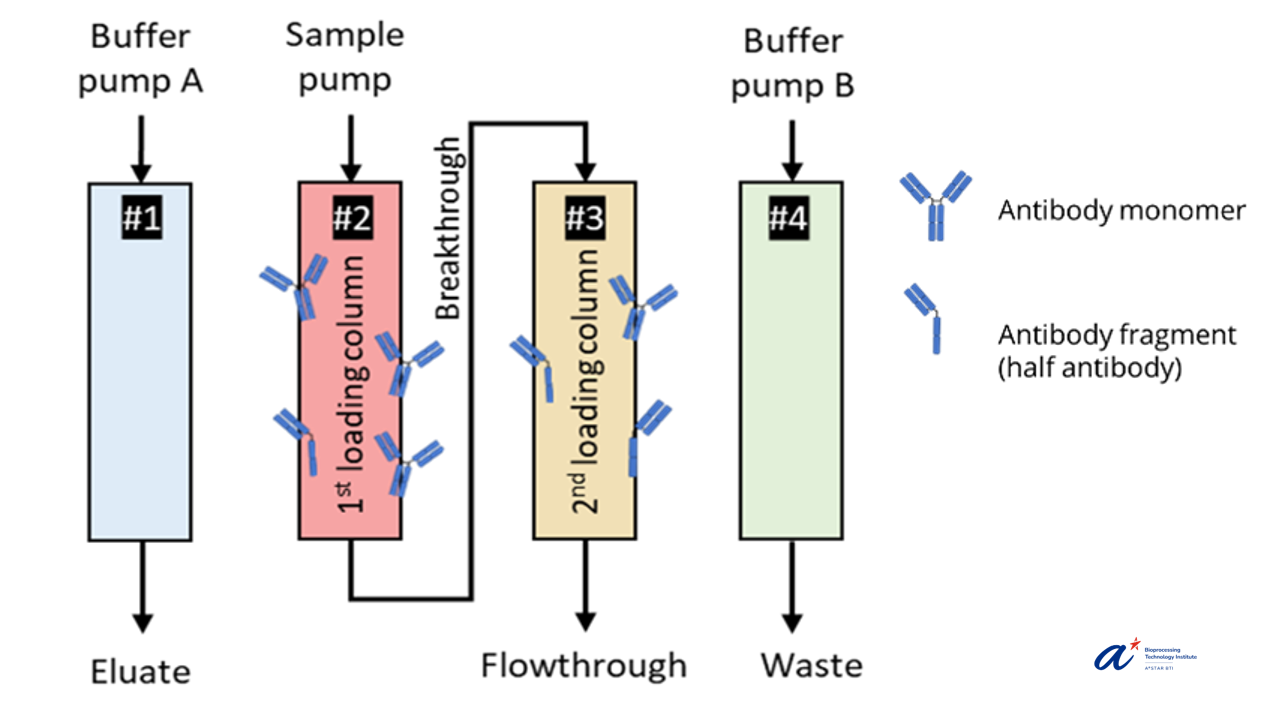
Figure 1. A 4-column chromatography for continuous biomanufacturing.
References
Zheng ZY, Chen AG, Dzulkiflie N, Sim LC, Tang WQ, Leong J, Ler WX, Leong D, Chng J, Manan NBA, Walsh I, Ng SK, Pennings M, Zhang W. Quality by Design in continuous bioprocessing: Investigating the impact of critical process parameters of multi-column chromatography during steady-state and transient phases. J Chromatogr A. 2025 Feb 8;1742:465649. doi: 10.1016/j.chroma.2024.465649. Epub 2024 Dec 31. PMID: 39755054.
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)