In-House Services
To advance its mission and vision to accelerate health technology innovations towards commercialization and adoption, SB will be availing a suite of in-house services to support this effort.
BIODESIGN PROCESS
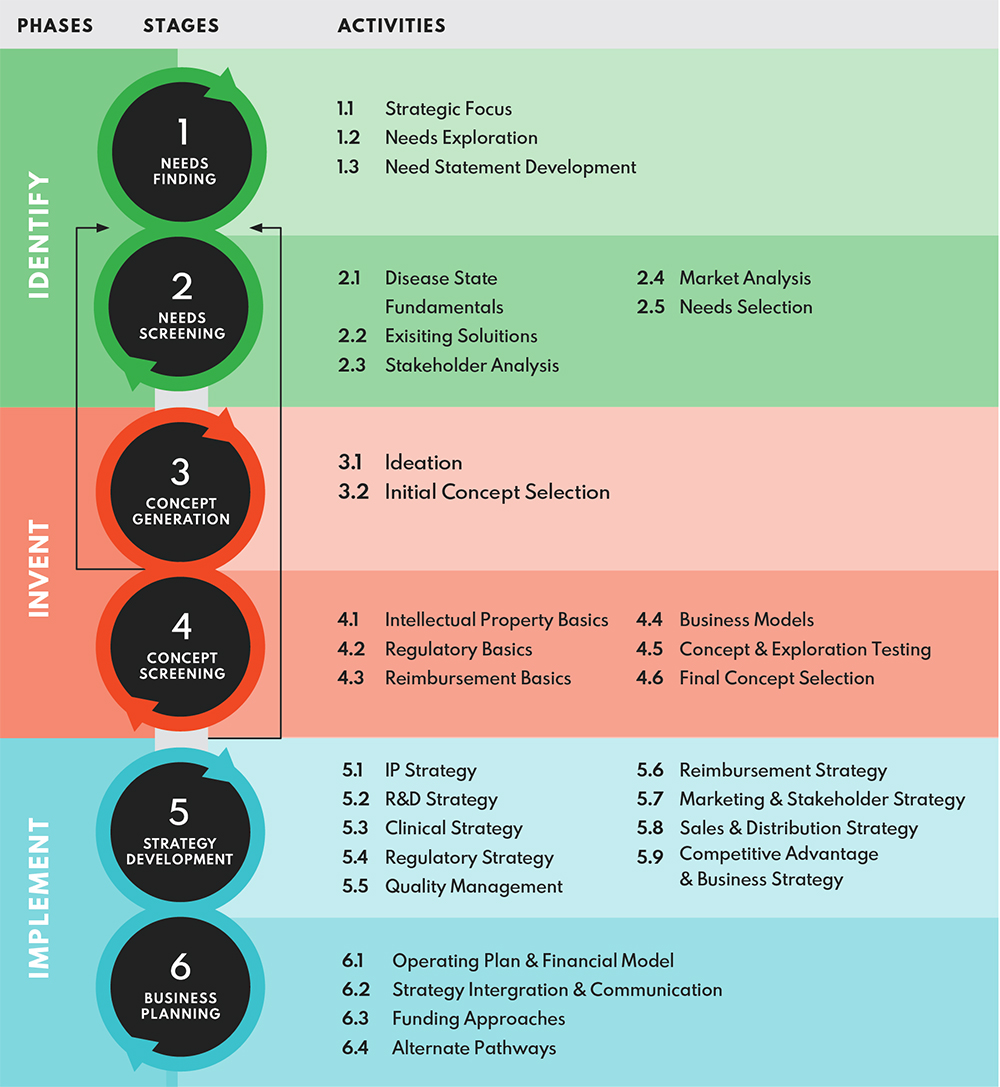
Reference: Adopted from Biodesign : The Process of Innovating Medical Technologies, Yock P. et al., ISBN13: 9781107087354
Needs finding is an integral part of the Biodesign process which allows a patient journey to be charted from start to end, extending beyond the hospital into the community so as to create contextualized and impactful innovations. Every year, our Fellows are immersed in a specific clinical theme for 3 – 4 months, for which they would have witnessed first-hand how hospital procedures and operations are conducted in various countries such as the U.S., SG, China and Indonesia. These observations are further processed and stored in a needs database which may be accessed for a fee. Separately, a needs investigation and/or validation study may be commissioned according to your requirements.
The Singapore Biodesign Programme is housed at A*START Central, A*STAR’s open innovation lab. Over the years, SB has acquired a range of engineering assets and has set up a rapid prototyping space at the engineering lab at A*START Central — Tinkerlab. This space has been upgraded to improve the access of its equipment and engineering services to support the early engineering needs of healthtech projects. In particular, we provide an array of materials for brainstorming and more specialized equipment for prototyping/modification.
The second valley of death for getting a health technology innovation to market is at the implementation phase, for which speed of execution is of essence. For this, SB aims to assist project teams via a fee-for-service approach to de-risk their projects via either of the following:
- Market Validation and KOL identification, especially in China, Indonesia, Singapore and the U.S.
- Preliminary business model scoping and planning
- Preliminary health technology assessment and pricing
- Sourcing & curation of vendors in our extended network in areas such as clinical trials, regulatory, intellectual property and reimbursement
For an overview, please download our in-house services brochure here.
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM



