High-density red blood cell production from stem cells in bioreactors
Science
Blood is a resource that is often overlooked in its importance. Blood transfusions are a common and often lifesaving procedure and are vital for accidents, surgeries, and cancer treatment. However, the global blood supply is both insufficient and irregular; for instance, the Covid-19 pandemic left many healthcare institutions with only a few days’ worth of blood as blood drives around the world were cancelled. Thus, research has focused intensely on shoring up this gap by unlocking the means of producing universal (O-negative) red blood cells (RBCs) at industrial scales. One of the most exciting solutions is human induced pluripotent stem cells (iPSCs) – cells that, given the correct signals, can transform into any cell type, including the red blood cells that are essential for modern medicine. However, blood transfusions require trillions of cells, a number that current manufacturing methods simply cannot produce cheaply. Our research aims to push the boundary of these production methods and reaching the highest density of RBCs achieved in a stirred bioreactor.
Societal Impact
A single transfusion unit of blood contains two trillion cells (2x1012). Most methods developed thus far are two-dimensional and rely on expensive media, containing cytokines and growth factors that stimulate stem cells to transition to RBCs. Hence, when more cells are needed, more physical space, manpower and growth factors are necessary. This leads to higher production cost. Current estimates place the cost of an iPSC-derived RBC transfusion unit at a minimum of USD 8000, whereas a unit of donated blood can be valued at around USD 500. Therefore, it is vital to develop processes that can achieve extremely high densities (1x108 RBCs per mL) to minimize the usage of media and growth factors. Our group has published a series of papers detailing the conception of a 3D suspension platform for RBC production, effectively tackling space and manpower efficiency challenges of 2D models. Our most recent publication demonstrates, for the first time, bioreactor cultures of RBCs at up to 35 million cells per millilitre, or 3.5x107 RBCs per ml, bringing down the cost per transfusion unit and inching ever closer to the desired cell density goal.
Technical Summary
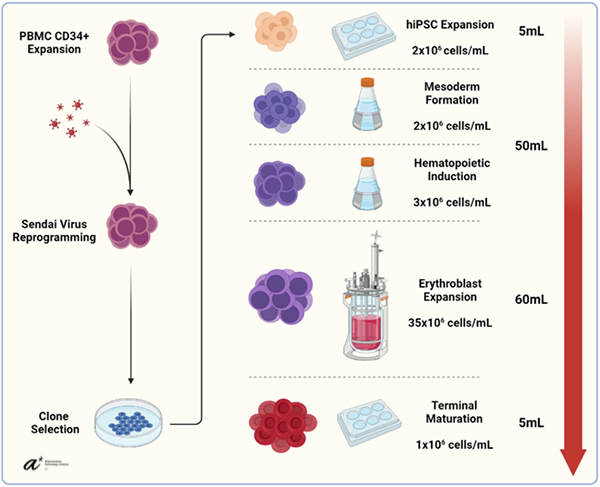
We first sought to obtain a suitable source of O-negative human iPSCs. To this end, 10 hiPSC (human iPSC) clones were generated using our microcarrier reprogramming platform from isolated CD34+ peripheral blood mononuclear cells. Research has shown that hiPSC clones possess varying degrees of differentiation ability even when isolated from the same donor. To identify the most promising clones, we performed tri-lineage differentiation and marker analysis, showing that 6 clones had higher relative expression of the mesoderm marker Hand1. Differentiating these clones down the erythroid lineage in our previously published microcarrier suspension platform allowed us to isolate the single best performing clone in terms of expansion potential and marker expression. This clone was then used for the bioreactor study. Early erythroblasts were obtained using shake flasks and were then inoculated into a stirred perfusion bioreactor, while spinner flasks inoculated at the same density served as controls.
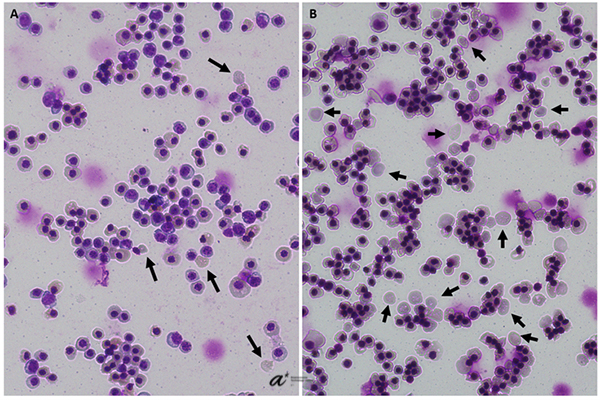
Perfusion was started one day post-inoculation, with the same amount of media being replaced for the spinners controls to simulate the perfusion. Opting for dynamic modulation of culture conditions, we altered the dissolved oxygen concentration in the bioreactor several times throughout the culture in response to slowing growth rates and managed to achieve a peak density of 3.5x107 cells/mL in the bioreactor, whereas spinner control densities stagnated at around 2x107 cells/mL despite equal medium exchanges. To our knowledge, this represents the highest erythroblast density achieved in a bioreactor format to date. We finally demonstrated that the erythroid cells obtained from this method were capable of enucleation and could carry oxygen effectively, albeit with a significant left-shift in equilibration curves, consistent with a higher expression of fetal hemoglobin noted in our previous papers using this differentiation method. Overall, our results should provide a meaningful milestone on the road towards affordable blood products and accelerate the development of novel solutions in the field.
References
SuE Y, Vassilev S, Lim ZR, Sivalingam J, Lam ATL, Ho V, Renia L, Malleret B, Reuveny S, Oh SKW. Selection of O-negative induced pluripotent stem cell clones for high-density red blood cell production in a scalable perfusion bioreactor system. Cell Proliferation. 2022. doi:10.1111/cpr.13218
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)