Novel bioreactor to reduce manufacturing cost of autologous cell therapy products
Science
T-cell therapy involves the use of efficacious immune cells (T-cells) to target and eliminate cancer cells to treat the disease. Healthy T-cells are first collected from the patient, primed outside of the body (in vitro) to recognize unique antigens on the cancer cells, and grown to a certain population prior to infusion back into the patient. The whole process can take weeks to months whereby the handling of cells can become laborious, tedious and prone to contamination risk. This leads to high manufacturing cost of the therapy and severely limits the number of therapies that can be produced and administered to patients. To address the high manufacturing costs associated with cell therapy development, our team worked with clinical collaborators to understand their process and limitations in current technologies to develop a novel bioreactor which would simplify the cell manufacturing process thus reducing risk of contamination, manhours and cost of manufacturing.
Societal Impact
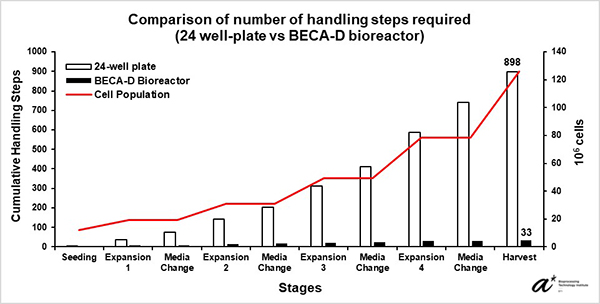
Our team has developed a novel bioreactor that aims not only to simplify the cell manufacturing process and facilitate parallel patient processing, but also to allow direct and seamless process transfer across therapy development phases from lab to manufacturing. The bioreactor would help reduce process related contamination risks and at the same time lower manpower requirements that eventually leads to improved manufacturing efficacy and lower manufacturing costs. The developed bioreactor was able to reduce handling steps by over 90% and consumption of consumables without affecting the quality of the cells for expansion of antigen-specific T-cells compared to 24-well plate. The use of the bioreactor thus has the potential to support more streamlined and cheaper immune cell therapy manufacturing process and reduce time in manufacturing process transfer, enabling more therapies to be produced for patients and accelerating immune cell therapies through clinical trials and market.
Technical Summary
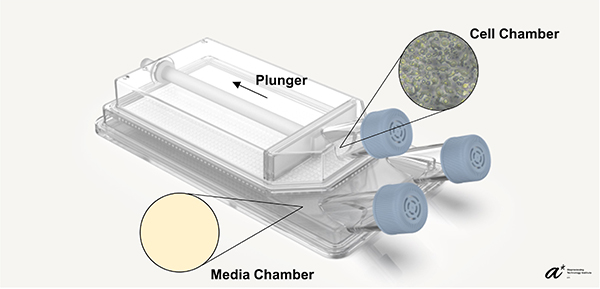
Maintaining cell culture density within an optimal range is essential for cell growth and function. To maintain consistent cell density, cells are usually required to be transferred from the original culture vessel to multiple new vessels. Our bioreactor, BECA (Bioreactor with Expandable Culture Area), was designed with a culture chamber that can be expanded in-situ. This feature allows consistent cell density to be maintained within the bioreactor while omitting the need for culture transfer across different vessels traditionally performed as the culture expands. BECA also has a separate media chamber, which facilitates media exchange and feeding without disturbance to the culture. The position of the media chamber, below the cell chamber, allows the culture to gain access to both bulk nutrient below and gaseous exchange above the culture. Resultantly, BECA was able to reduce handling steps and minimize disturbance to the culture during the cell culture process.
Our clinical collaborators in National Cancer Centre Singapore expanded Epstein-Barr Virus-Specific T-cells (EBVST) in 24-well plate, the process of which is not scalable as the culture process gets highly laborious as the culture expands (a culture of 100 million cells requires seeding into 100 individual wells). Due to EBVSTs’ sensitivity to culture conditions such as cell-cell contact and flow shear, most bioreactors whose expansion feature involves increase in culture volume and agitation (e.g., stirred tanks and culture bags) are unsuitable for EBVST culture. Our validation study has shown EBVST culture in BECA matches that in 24-well plate in terms of growth and performance while reducing handling steps by over 90%. The study concludes that BECA is able to support EBVST culture with lower manpower requirements compared to the 24-well plate leading to lower cost and higher efficiency in therapy development. With different immune cell therapies often requiring specific culture and therapy development needs, BECA with unique and functional features provides cell therapy manufacturers and developers an alternative to consider when selecting scale-out solutions for their needs.
References
Chen, S., Bin Abdul Rahim, A.A., Wang, WW. et al. In-situ scalable manufacturing of Epstein–Barr virus-specific T-cells using bioreactor with an expandable culture area (BECA). Sci Rep 12, 7045 (2022). https://doi.org/10.1038/s41598-022-11015-z
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM
.png?sfvrsn=1a7df424_3)