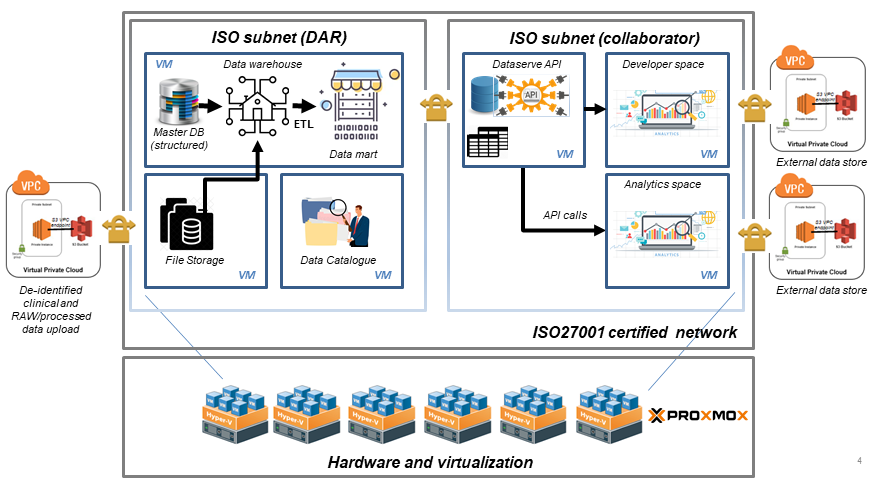
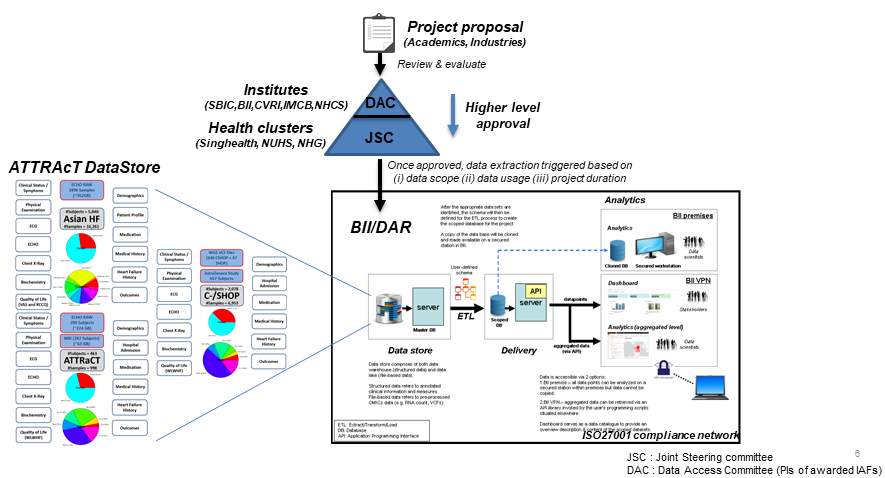
BioMedical Data Architecture & Repository

WONG Wing Cheong
 | WONG Wing Cheong Head of BioMedical Data Architecture & Repository Email: wongwc@a-star.edu.sg Research Group: BioMedical Data Architecture & Repository |
Wong Wing Cheong received his undergraduate degree in Computer Engineering from NTU. He joined A*STAR under the joint MSc in Bioinformatics from A*STAR/NUS and subsequently completed his Dr. Rer. Nat (suma cum laude) in Computer Science from Leipzig University. He has been a Principal Investigator with BII since 2014 and is currently the Head/Principal Investigator of the BioMed DAR group since 2019.
Group Members
| Senior Scientist | TAN Ming Zhen |
| Senior Scientist | FUN Max |
| Senior Research Officer | LIM Aloysius |
| Senior Research Officer | KWOK Jia Hui |
| Senior Research Officer | LEE Jiong Yao John |
| Research Officer | DONG Jiahui |
| Research Officer | KOH Ziying |
TAN MING ZHEN
 | TAN Ming Zhen Assistant Principal Investigator Email: tan_ming_zhen@a-star.edu.sg Research Group: BioMedical Data Architecture & Repository |
Dr Tan Ming Zhen graduated from the National University of Singapore in 2012 with a B.Eng. (Hons) and B.Soc.Sci (Hons). He then moved on to the NUS Graduate School for Integrative Sciences to pursue his PhD in Engineering, which he obtained in 2017. During his PhD, he worked on several research projects involving mathematical modelling, image processing, and statistics. After completing his PhD, he joined a local FinTech start-up as the Head of Technology, developing the code base for passive investment. In 2021, Dr Tan Ming Zhen joined the Bioinformatics Institute as Assistant Principal Investigator, where he is currently researching data management support and privacy-protection technologies.
Research Interests
Dr Tan has previously worked on the diffeomorphic metric mapping of brain surfaces and volumes, as well as keeping a real-time dashboard for manipulating datasets, visual data exploration tools, and implementing statistical tests. His current area of study is synthetic data and its potential use as a privacy-protection tool. More specifically, he is researching methods for generating high-fidelity synthetic data from real-world data sets, and hopes to improve their potential as surrogates for code development and research.
Group Members
| Senior Principal Scientist | WONG Wing Cheong |
| Senior Scientist | FUN Max |
| Senior Research Officer | LIM Aloysius |
| Senior Research Officer | KWOK Jia Hui |
| Senior Research Officer | LEE Jiong Yao John |
| Research Officer | DONG Jiahui |
| Research Officer | KOH Ziying |
A*STAR celebrates International Women's Day

From groundbreaking discoveries to cutting-edge research, our researchers are empowering the next generation of female science, technology, engineering and mathematics (STEM) leaders.
Get inspired by our #WomeninSTEM